Case Presentation:
You are on CCU nights when the ED calls you regarding a cardiac arrest patient. He is a 57-year-old male with no known past medical history. He was with his daughter at a resturant when he suddenly became unresponsive without a pulse. Since his daughter is a nurse, she started high-quality compressions immediately. When EMS arrived, they immediately intubated and shocked the patient for notable ventricular fibrillation. He received 5 shocks, 2 doses of epinephrine, and 1 dose of amiodarone before achieving ROSC in the ED. Unfortunately, the patient was not responsive post-arrest, so targeted temperature management was initiated.
Bedside ultrasound showed an LV with regional wall motion abnormalities but no pericardial effusion. Initial vitals: temp 97.7° F, HR 50, RR 22, BP 108/69, SpO2 97% on 40% FIO2. Initial labs were noteworthy for troponin of 30,000, BNP of 18, Cr. 1.47, and WBC 15.3. An EKG post-ROSC was attained. CXR confirmed ETT placement, KUB confirmed OGT placement.
Ask Yourself:
When do you consider coronary angiogram in patients who have arrested?
Which patients should be on targeted temperature management (TTM)? How soon after cardiac arrest should a patient begin TTM? If a patient is receiving TTM, how long should they be cooled?
The family asks about his prognosis. What should you tell them?
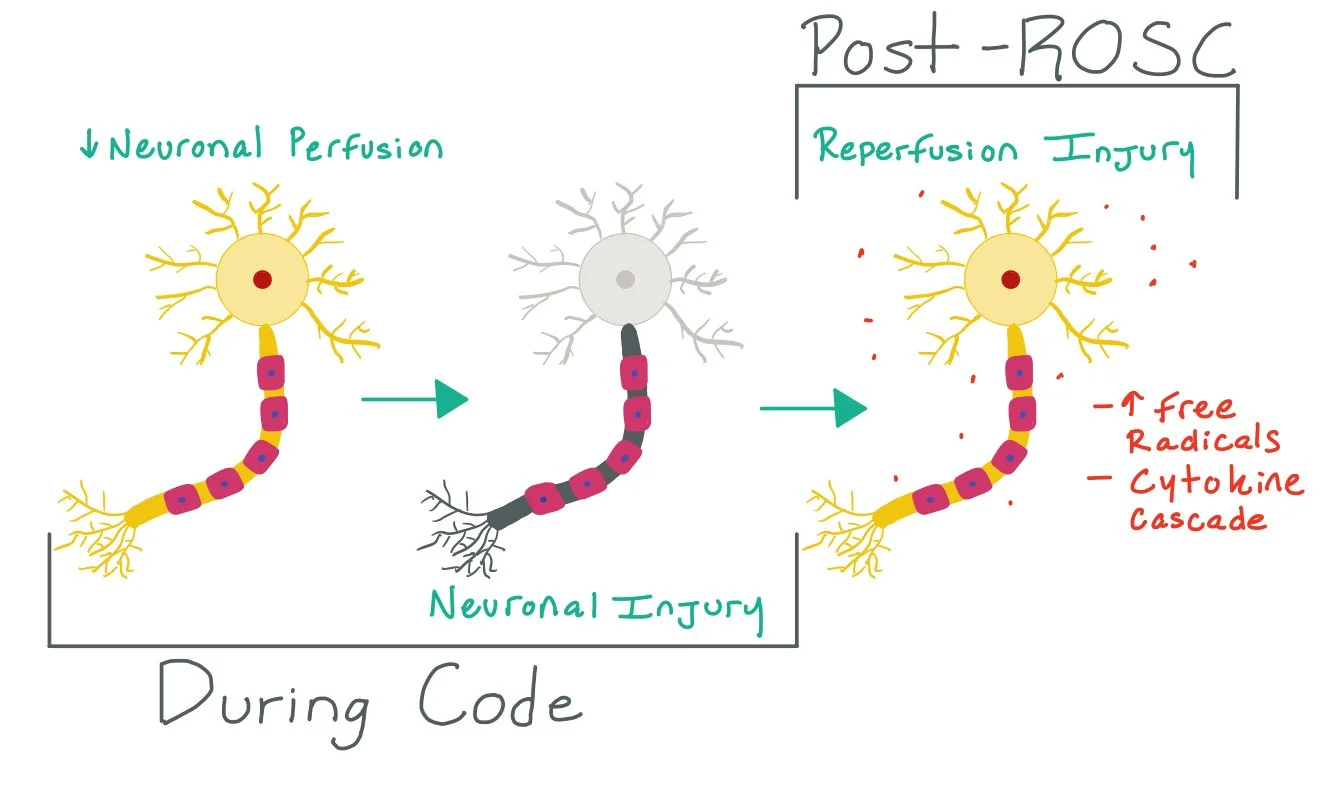
The picture above demonstrates how cardiac arrest causes decreased neuronal perfusion, which leads to neuronal injury. Once the patient has been stabilized post- return of spontaneous circulation (ROSC), there is a high risk for reperfusion injury that can lead to neuronal injury. TTM can help mitigate these risks by decreasing the metabolic demand of the neuron.
Background:
Whether patients have in- or out-of-hospital cardiac arrests (290,000 vs >340,000 in the US, respectively), the chances of survival are low (4.6-14.6%). Of the surviving patients, it is estimated that ~7% of patients achieve functional neurologic recovery. These low rates of recovery highlight the importance of ensuring our patients have appropriate post-arrest care so that we can give them the best chances of meaningful surivival and neurologic recovery.
Targeted temperature management (TTM) is a method of therapeutic hypothermia, which has been shown to improve neurologic outcomes and short and long-term mortality. For the past 20+ years, there have been multiple trials regarding active temperature management post-arrest to help prevent secondary brain injury in patients who are unable to follow commands and remain unresponsive after cardiac arrest.
The higher the temperature above 37.5 degrees Celsius, the increased risk of severe disability, coma, or persistent vegetative state.
TTM mechanism of action:
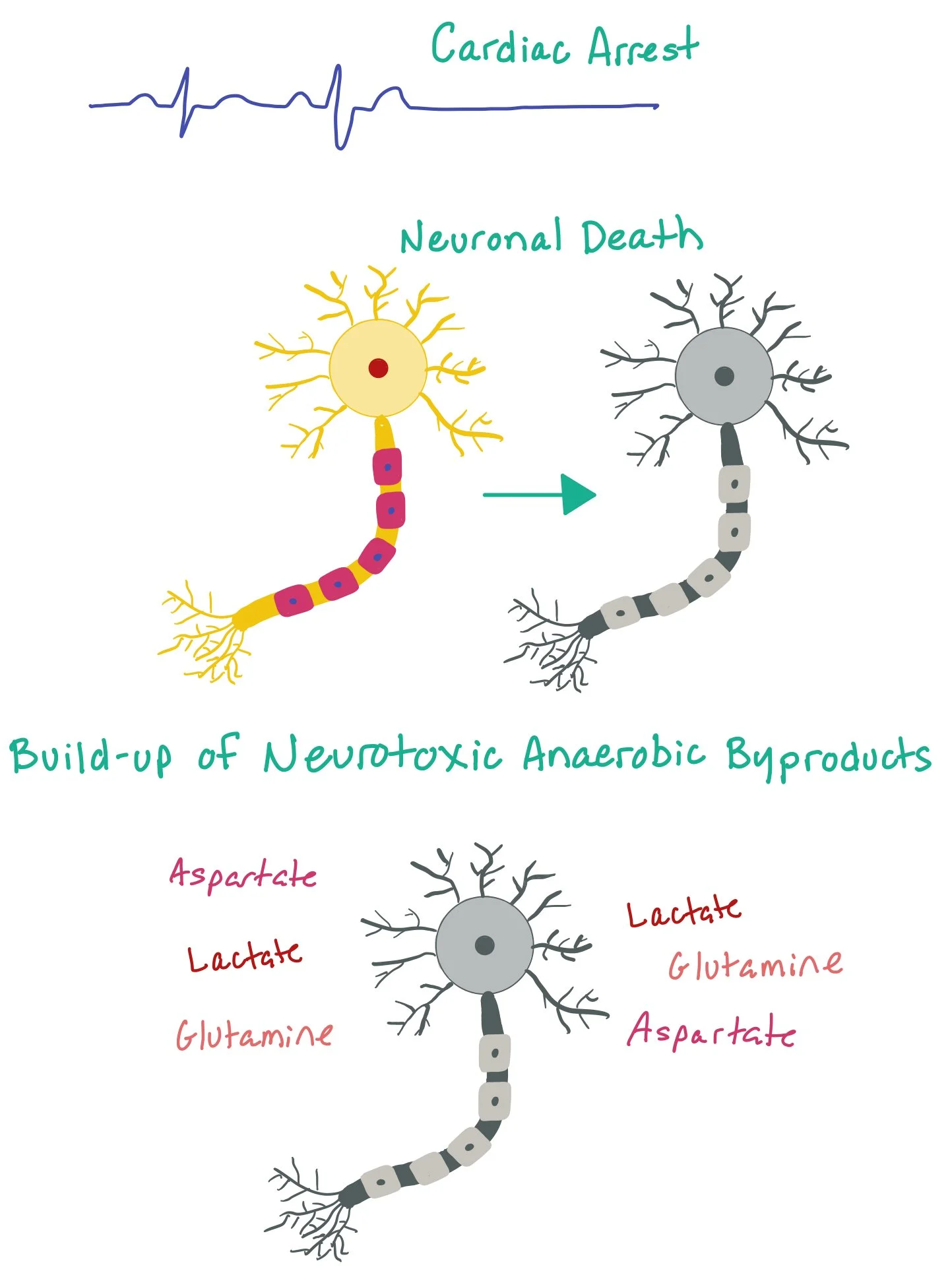
Limiting neuronal death: When patients arrest, the brain can undergo cerebral ischemia due to lack of perfusion. The cerebral ischemia causes an accumulation of neurotoxic anaerobic metabolism byproducts (such as lactate, aspartate, glutamine, and other excitatory neurotransmitters) that cause excitotoxicity and neuronal damage (as pictured here). By cooling the patient, we are slowing down the metabolic rate and thus limiting the toxic cellular byproducts that cause neuronal injury.
Decreasing free radical production: Once a patient gets ROSC, the cerebral reperfusion can cause increased free radical production (specifically with hydrogen peroxide, superoxide, nitric oxide, and hydroxyl radicals). The more free radicals present, the more harder it becomes for the body’s physiologic antioxidant defense mechanisms to protect from these toxic metabolites. This ultimately causes oxidative injury to the lipids, proteins, and nucleic acids in the body and neuronal damage. By cooling, we are hoping to decrease nitric oxide production and superoxide formation at lower temperatures
Decreasing the inflammatory response: Again, cerebral reperfusion post ROSC can cause an exaggerated inflammatory cascade that leads to high levels of destructive cytokines. The more cytokines, the more cerebral injury. TTM hypothermia impairs the secretion of pro-inflammatory cytokines while decreasing leukocyte migration and phagocytosis
TTM, TTM2, and ICECAP Trials:
The particulars of cooling, especially the temperature to which the patient should be cooled and duration of cooling, have been heavily debated. The TTM trial in 2014 explored cooling to 33° C vs 36° C, but found that cooling to lower temperatures of 33° C was not associated with reduction in all-cause mortality or improvement in neurologic outcomes when compared with 36° C. The TTM2 trial in 2021 found that targeted hypothermia was not associated with improved surivival, functional outcomes, or quality of life measuremes compared to active maintenance of normothermia. In fact, TTM2 found that arrythmias were more common in the hypothermia (33° C) group. According to the current 2023 AHA Adult Advanced Cardiovascular Life Support update, regardless of exact temperature target (32° C to 37.5° C), preventing fever with continuous temperature monitoring is crucial in patients who remain unresponsive post-arrest, regredless of arrest location or presenting rhythm. Notably, patients who have spontaneous hypothermia after ROSC should not routinely be actively or passively rewarmed faster than 0.5° C per hour.
As opposed to the TTM and TTM2 trials that look at temerpature to cool, the ongoing ICECAP study (Influence of Cooling Duration on Efficacy in Cardiac Arrest Patients) has been focused on evaluating duration of cooling in patients with both shockable and unshockable rhythms. It is important to note that patients who have severe trauma, active bleeding, profound bradycardia, or other electrical cardiac instability may require faster rewarming.
In addition to TTM, these patients should also receive EEG to ensure they are not having active seizures.
When to Cath…
According to the AHA 2023 Adult Advanced Cardiovascular Life Support update, coronary angiogram should be performed emergently for all patients with cardiac arrest who have suspected cardiac cause of arrest and ST-segement elevation on EKG. Patients who do not have STEMIs but have shock, electrical instibility, signs of significant ongoing myocardial damage, or ongoing ischemia can also be considered for emergent cath.
Notably, patients who arrest secondary to STEMIs tend to have better rates of neurologic recovery. The faster these patients are revascularized, the more mortality benefit they receive.
It should be noted that EKGs done immediately post-ROSC may have ST-elevations which is thought to be due to myocardial ischemia from no or low-flow coronary perfusion during the cardiac arrest. Many studies have shown that there is a higher probability of having a false positive STEMI on EKG within the first 7 minutes post-ROSC. As such, repeat EKGs should be performed > 8 minutes post-ROSC if the initial EKG was concerning for a STEMI to help better differentiate whether or not the patient had a true MI which caused the arrest. When in doubt, cardiology should be called and a serious discussion should take place about taking the patient to the cath lab for coronary aniography.
Study in JAMA from Baldi et al. showing how false-positive ECG findings and their correlation to timing post-ROSC.
Patient Selection for TTM
AHA guidelines recommend treating unresponsive adult patients with cardiac arrest, regardless of location of arrest, with temperature management targeting a temperature between 32° C and 36° C for 24 hours of therapy. Note: normal core body temperature range is 36.1 - 37.2° C; hypothermia is defined as temperature lower than 35° C.
Phases of TTM
Induction: Achieve a core temperature of 32 to 34° C as soon as possible. Note: Some centers will just keep <36° C to prevent any fevers as the data around exact cooling temperature is unclear. Studies investigating the optimal time of induction found no increased benefit for cooling interventions prior to hospital arrival
Cooling methods:
Conventional cooling techniques: cold saline infusion (up to 2 L), ice packs (best for induction, not as reliable for maintenance)
Surface cooling systems: blankets or pads wrapped around patients circulating cool fluid or air
Core cooling systems: central catheter
*** Note: AHA recommends against the use of prehospital cooling with rapid infusion of large volume cold IV fluids (no effect on neurologic outcome or mortality with increased risk of pulmonary edema and rearrest)
2. Maintenance: The goal is to maintain the temperature for 24 hours.
Temperature monitoring:
Gold standard: pulmonary artery catheter (provides most accurate, real-time measurement)
Esophageal temperature (average lag time of 5 min)
Rectal temperature (average lag time of 15 min)
Bladder temperature (average lag time of 20 min)
Peripheral sites are inaccurate and should not be used
3. Rewarming: This is done at a controlled rate of 0.2 to 0.5° C/hour
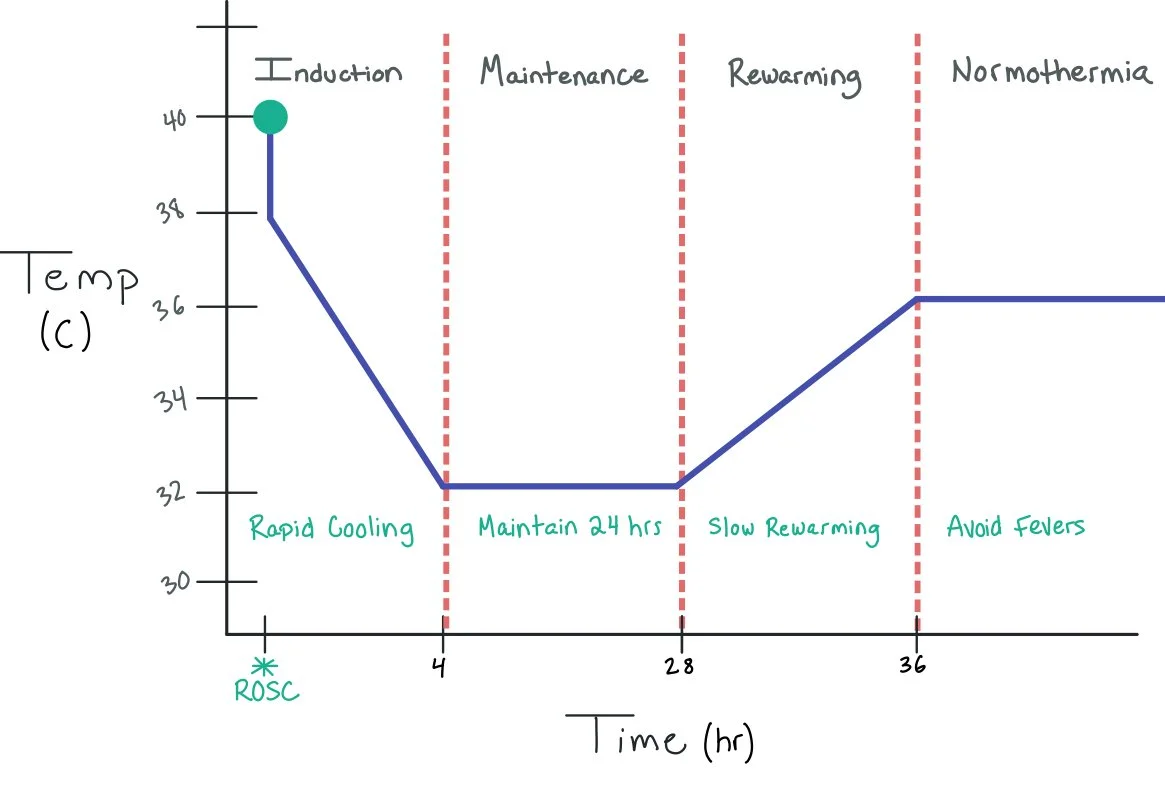
The picture above shows the different stages of targeted temperature management, including induction, maintenance, rewarming, and normothermia. ***Note: specific temperatures and exact timing of phases can be variable depending on the institution.
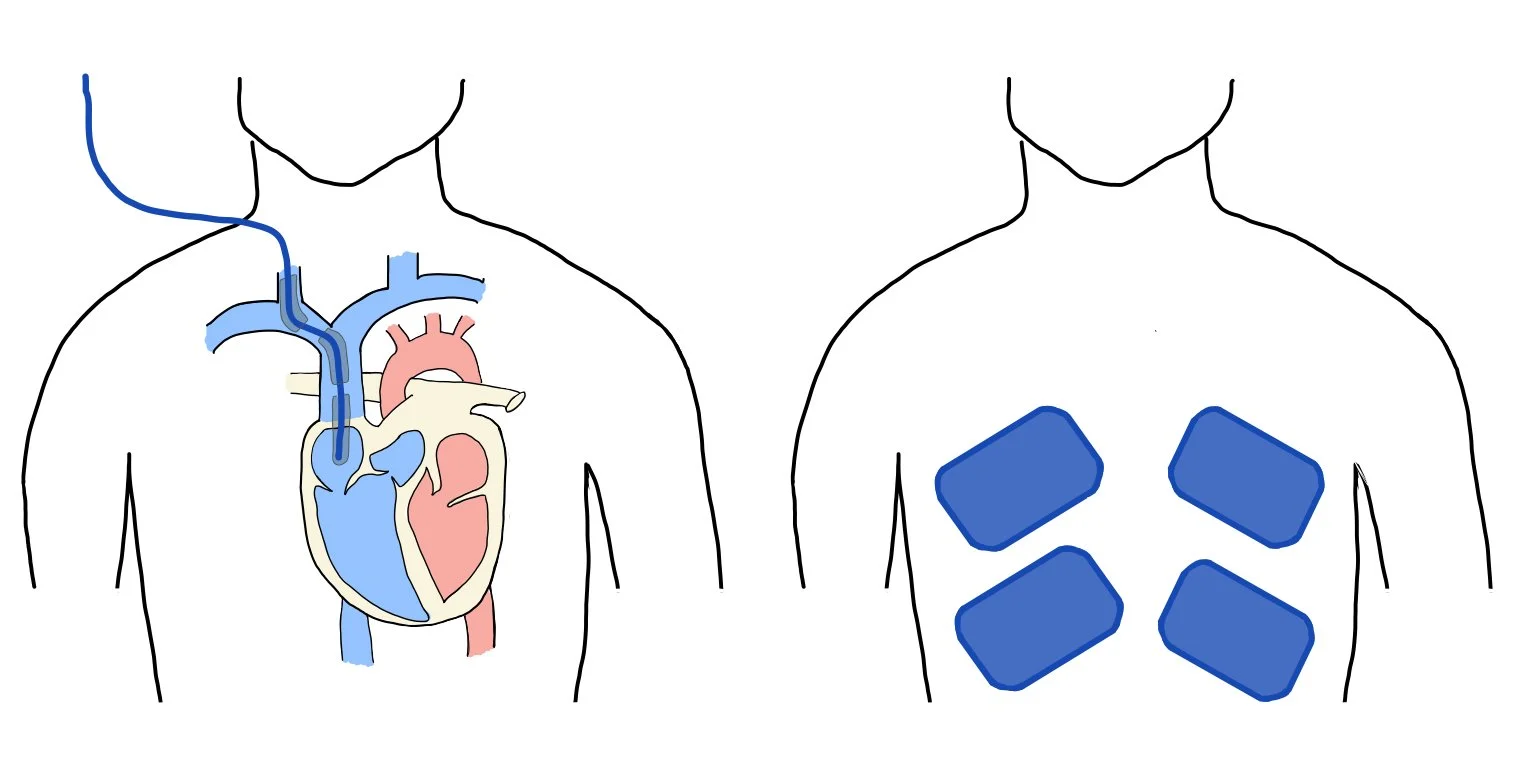
The picture above depicts two different methods for cooling patients, which include central catheter cooling (left) and surface cooling systems (right).
This is a picture of a central cooling catheter. If you look closely, you can see the balloon around the catheter, which is filled with cool fluid. Note that this method does not require actual infusion of cold saline — rather, the saline cools the catheter (and thus the blood) and is recycled throughout the catheter system.
Absolute Contraindications:
Hemorrhagic stroke
GCS >8
Uncontrolled bleeding
Uncontrolled hemodynamically unstable rhythms
Cardiac arrest 2/2 trauma
Exclusion criteria: >8 hours from arrest
*** Note: previous studies have attempted to start the hypothermia protocol within 1 hour, with one excluding patients at >3 hours
Relative Contraindications:
Thrombocytopenia (< 50,000)
Coagulopathy
Prolonged cardiac arrest (>60 min)
Refractory hypotension despite fluid vasopressor support
Pregnancy
Recent major surgery (especially intracranial)
Neurologic Tests to Order
This chart includes some of the main tools we use to test patient’s neurologic function, from the neurologic exam, to measuring the patient’s brainwaves, to actual neuroimaging.
Pupillary Light / Corneal Reflex:
Considerations: Oculocephalic/vestibulocochlear reflex, gag, and cough reflexes are not reliable indicators. Early-onset myoclonus previously thought to be a poor predictor; however not reliable
Results Concerning for Poor Prognosis: Absent bilateral pupillary light reflex >72 hours from ROSC. Absent bilateral corneal reflex can NOT be used alone (unreliable predictor)
EEG:
Considerations: EEG may identify patients more likely to have a good outcome (presence of continuous background, presence of EEG reactivity)
Results Concerning for Poor Prognosis: Background suppression, Burst suppression (+/- periodic discharges), >72 hours from ROSC and rewarming, in the absence of confounders (sedation, hypothermia) is a moderately reliable predictor of poor outcome when the overall clinical picture is consistent with severe diffuse brain injury
Somatosensory Evoked Potential (SSEP): Assess integrity of the dorsal column-lemniscal pathway (transmission of sensory info)
Considerations: Less affected by sedatives and opioids compared to EEG. Confirmation of the presence of responses at Erb’s point and the cervical spine is a prerequisite because extracranial injury, including at the level of the cervical spine with hanging or other trauma, may abolish the N20 response
Main confounder: Hypothermia can abolish the N20 response. Consider obtaining SSEPs when clinical and radiographic findings are insufficient for accurate neuroprognostication
Results Concerning for Poor Prognosis: In the absence of spinal cord injury (confounder), bilateral absence of N20 potentials (generated in the primary somatosensory cortex following electrical stimulation of the median nerve) is a reliable predictor of poor outcome when the overall clinical picture is consistent with severe diffuse brain injury.
CT:
Considerations: CT obtained on admission is to rule out a neurologic etiology for cardiac arrest, but may early-on demonstrate hypoxic ischemic brain injury. CT must then be repeated at least 48 hours from ROSC to assess extent of injury.
Results Concerning for Poor Prognosis: Diffuse loss of gray-white differentiation in cortical and deep structures + sulcal effacement >48 hours from ROSC is a moderately reliable predictor of poor outcome when the overall clinical picture is consistent with severe diffuse brain injury
MRI:
Considerations: Consider with normal CT; DWI and FLAIR show hyperintensities with corresponding hypointensities on ADC. Other conditions can result in restricted diffusion, including some in a diffuse pattern, such as hyperammonemic encephalopathy and seizures. MRI may demonstrate at least moderate accuracy for the prediction of good long-term outcome (when normal)
Results Concerning for Poor Prognosis: Diffuse extensive involvement in the bilateral cerebral cortex and deep gray matter 2-7 days from ROSC is a moderately reliable predictor of poor outcome when the overall clinical picture is consistent with severe diffuse brain injury
Interpretation:
If findings from the following categories are present that suggest a poor prognosis, the likelihood and certainty of poor prognosis depends on the collective results of the clinical, radiographic and electrographic findings.
If there are no reliable or moderately reliable predictors of poor outcome present then it would be considered an indeterminate prognosis (longer period of observation is necessary to better elucidate the long-term outcome)
Long-term outcomes with indeterminate prognosis:
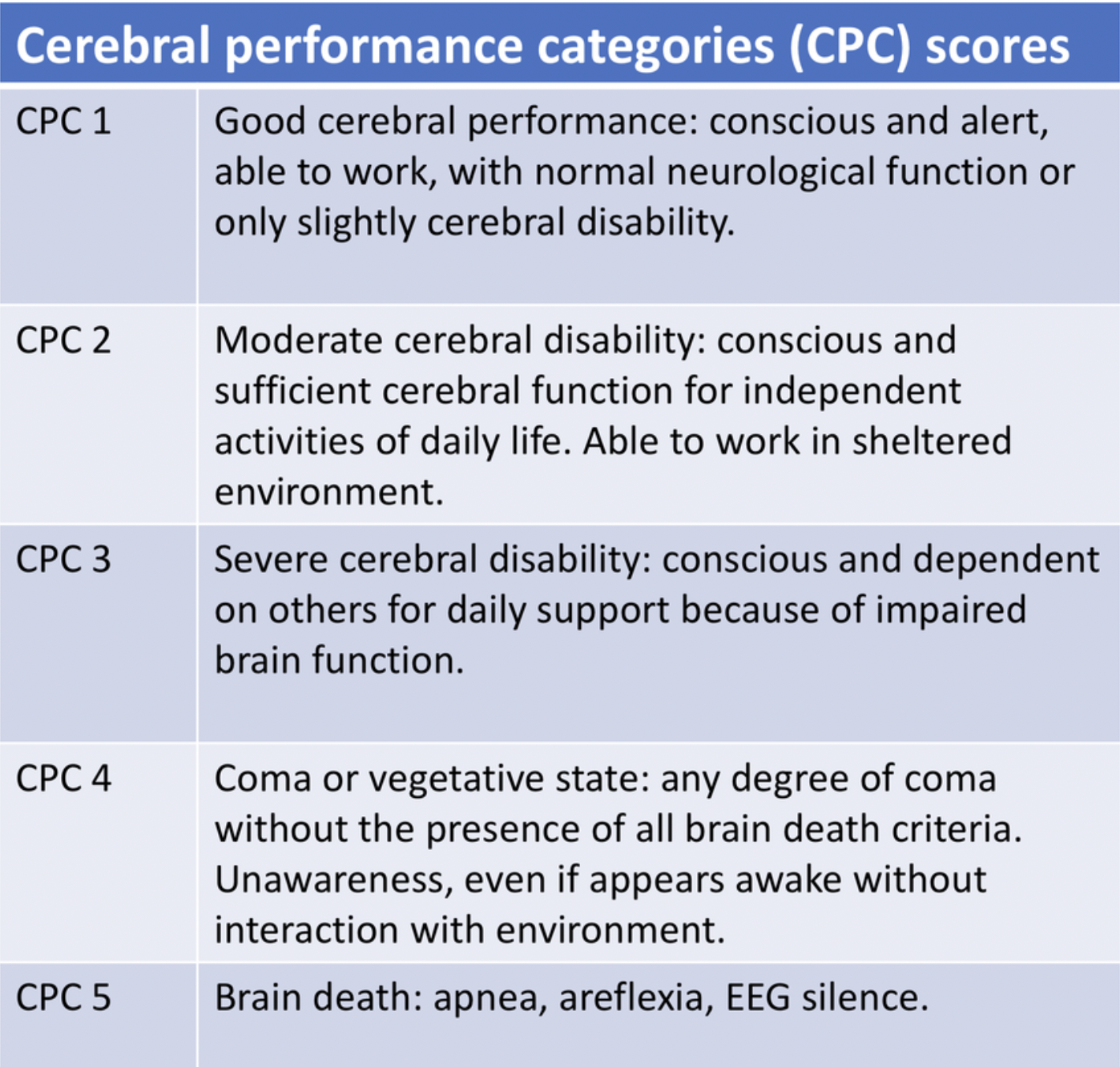
Good outcomes (defined as cerebral performance category 1-2):
49% after 6 months; 42% after 5.1 years
Most who had a good outcome regained consciousness within 12 days, small percent required up to 25 days post-ROSC
GCS <9 when sedation was weaned at 72 hours post-arrest
30% with good outcome with given more time to regain consciousness
We also use the Cerebral Performance Categories (CPC) to help us neuroprognosticate.
Neuroprognostication
~80% of patients remain comatose post-cardiac arrest (result of hypoxic-ischemic brain injury). A small percentage succumb to multiorgan failure, but the most frequent cause of death post-arrest is withdrawal of life-sustaining therapies. Deciding whether or not to continue aggressive treatments should be based on the severity of neurologic injury, the potential for neurologic recovery, and how the expected deficits fit within individual perspectives of a tolerable level of disability.
Neuroprognostication should be deferred in the early post-arrest period to exclude confounders (sedatives, home meds, illicit drugs). Deferral of assessment of neurologic prognosis of comatose survivors of cardiac arrest for at least 72 hours following ROSC in patients not treated with TTM and at least 72 hours in patients who received TTM following rewarming. Note: Persistent of coma past 72 hours in the ICU may not be equated with poor neurologic prognosis!!
Additionally, in order to make these prognostications, patients must be off sedation. Duration of medication half-life, amounts infused, hepatic and renal function, and drug interactions all need to be taken into consideration.
Other factors, such as seizures, sepsis, organ failure (i.e. hepatic encephalopathy, renal failure, etc), and deliruim must also be taken into consideration.
After 72 hours, you should use a multimodal approach, such as clinical exam, imaging, electrophysiologic studies, to help determine prognosis. The exact combination depends on various factors, like accessibility, perceived utility, institutional protocols, patient-specific factors, ongoing confounders, safety, etc.
Many studies have found that withdrawal of care by physicians due to perceived lack of recovery is the greatest risk for poor outcomes. Many times, patients with neurologic injury post-arrest will actually make significant neurologic improvement; however, this takes time. Unfortunately, we as the care team may be too quick to prognosticate. All this said, it is crucial that we include our neuro-intensivists early so that we ensure our patients are getting the best care possible.
SHIVERING:
Shivering:
When patient’s are being cooled, we need to ensure they do not shiver, a natural reflex by the body to maintain normothermia. Shivering, which is a rhythmic contraction and relaxation of skeletal muscles, increases O2 consumption and heat dissipation, which is counter-intuitive to cooling. Therefore, we have many different methods to prevent this from happening. Here, we picture a figure from University of Illinois in Chicago of their shivering protocol, which will most likely vary between different institutions.
Complications / Interventions:
Complications:
25% decrease in cardiac output
Increase myocardial O2 demand (from compensatory catecholamine production)
Increased PVR
Bradycardia
Arrhythmia (with temp below 30° C)
Hypothermia-induced diuresis (decreased ADH) → can lead to electrolyte abnormalities
Hypothermia causes intracellular electrolyte shifts (mainly Mag, K, and phos)
Increased risk ratio for PNA and sepsis (with suppressive effects on the immune system)
Left shift of oxygen-hemoglobin dissociation curve → less O2 available → metabolic acidosis
Drug toxicity from decreased metabolism
Decreased PaCO2 production → need to appropriately account for and adjust with vent settings
Back to the Case:
1. When do you consider coronary angiogram in patients who have arrested?
You should always consider coronary angiogram in all patients who arrest, as acute coronary syndrome needs to be ruled out as a primary case of the patient arresting. It is important to note that there is a higher likelihood of false-positive STEMIs on EKGs within the first 7 minutes post-ROSC. As such, it is important to repeat the EKG at regular intervals > 8 minutes to determine if there are an changes in the EKG (both confirming or disproving STEMI). Regardless, always call your cardiology fellow if you aren’t sure!! Patients who arrest due to ACS tend to do significantly better with coronary catheterizations than those who do not undergo the procedure and stenting.
2. Which patients should be on targeted temperature management (TTM)? How long should they be cooled?
AHA guidelines recommend treating all unresponsive adult patients who survive cardiac arrest, regardless of location of arrest, with temperature management targeting a temperature. If the patient arrests on the field, they should be cooled once they reach the hospital to temperatures between 32° C and 36° C for 24 hours of therapy. After 24 hours, the patients should be rewarmed at a rate of 0.2 to 0.5° C/hour
*** Make sure to get your neuro-intensivests on board ASAP, especially if you aren’t sure whether or not the patient should be cooled!!
3. The family asks about his prognosis. What should you tell them?
When neuroprognosticating, we have to be very careful. Many studies have found that withdrawal of care by physicians due to perceived lack of recovery is the greatest risk for poor outcomes. Many times, patients with neurologic injury post-arrest will actually make significant neurologic improvement; however, this takes time. Unfortunately, we as the care team may be too quick to prognosticate. All this said, it is crucial that we include our neuro-intensivists early so that we ensure our patients are getting the best care possible. No neuroprognostication should be done until at least 72 hours post-ROSC.
Further Learning:
Resident responsibilities:
TTM should be considered for all patients after ROSC, assess for following verbal commands and know the contraindications.
Best approach is early TTM. You must first stabilize the patient, but the next thought should be TTM. Studies have had goals of starting within 60 minutes of ROSC.
Depth of cooling remains controversial, initial goal of 32 – 34°C is reasonable, but may be adjusted based on the clinical context and if adverse events are encountered during deeper cooling. Most importantly, AVOID FEVER!
The brain is vulnerable even after the 24 hours of TTM, goal remains to avoid fever for first 72 hours.
Neuroprognostication is an imperfect science, but has lasting effects on family decision making. Careful and timely assessments are important in determining prognosis, these are often longitudinal assessments that can extend well beyond the initial 72 hour assessments.
High yield trials and resources for further reading:
TTM and TTM2 Trials, which debate the appropriate temperature for TTM
Trial showing how post-ROSC EKGs can show false-positives for STEMI!
Remember to think of TTM early! Withdrawal of care by physicians due to perceived lack of recovery is the greatest risk for poor outcomes. Many times, patients with neurologic injury post-arrest will actually make significant neurologic improvement; however, this takes time. Unfortunately, we as the care team may be too quick to prognosticate. All this said, it is crucial that we include our neuro-intensivists early so that we ensure our patients are getting the best care possible.
How’d we do?
The following individuals contributed to this topic: Joey Ballard, MD; Fatan El Ammar, MD; Dustin Fraidenburg, MD
Resources
Omairi AM, Pandey S. Targeted Temperature Management. [Updated 2023 Jun 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK556124/
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002 Feb 21;346(8):557-63. doi: 10.1056/NEJMoa003289. PMID: 11856794.
Perman SM, Bartos JA, Del Rios M, Donnino MW, Hirsch KG, Jentzer JC, Kudenchuk PJ, Kurz MC, Maciel CB, Menon V, Panchal AR, Rittenberger JC, Berg KM; American Heart Association Emergency Cardiovascular Care Committee, Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, and Stroke Council. Temperature Management for Comatose Adult Survivors of Cardiac Arrest: A Science Advisory From the American Heart Association. Circulation. 2023 Sep 19;148(12):982-988. doi: 10.1161/CIR.0000000000001164. Epub 2023 Aug 16. PMID: 37584195.
Duong H, Patel G. Hypothermia. [Updated 2024 Jan 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545239/
Bernard SA, Smith K, Cameron P, Masci K, Taylor DM, Cooper DJ, Kelly AM, Silvester W; Rapid Infusion of Cold Hartmanns (RICH) Investigators. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation. 2010 Aug 17;122(7):737-42. doi: 10.1161/CIRCULATIONAHA.109.906859. Epub 2010 Aug 2. PMID: 20679551.
Vaity C, Al-Subaie N, Cecconi M. Cooling techniques for targeted temperature management post-cardiac arrest. Crit Care. 2015 Mar 16;19(1):103. doi: 10.1186/s13054-015-0804-1. PMID: 25886948; PMCID: PMC4361155.
Choi HA, Ko SB, Presciutti M, Fernandez L, Carpenter AM, Lesch C, Gilmore E, Malhotra R, Mayer SA, Lee K, Claassen J, Schmidt JM, Badjatia N. Prevention of shivering during therapeutic temperature modulation: the Columbia anti-shivering protocol. Neurocrit Care. 2011 Jun;14(3):389-94. doi: 10.1007/s12028-010-9474-7. PMID: 21210305.
Neuroprognostication After Cardiac Arrest. Kromm, Julie et al. CHEST Critical Care, Volume 2, Issue 3, 100074
Nolan JP et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med. 2021 Apr;47(4):369-421. doi: 10.1007/s00134-021-06368-4. Epub 2021 Mar 25. PMID: 33765189; PMCID: PMC7993077.