Case Presentation:
You are working overnight in the CCU when you admit a new patient from the ED. Your patient is a 68 year-old male with a past medical history of HTN, HLD, and CAD with a past PCI of the RCA ten years ago. He presented earlier in the evening with substernal chest pain radiating to the left shoulder which started three days ago. The pain was relieved by nitroglycerin in the ED. His ECG on presentation showed ST-depressions and TWI in the anterior and lateral leads. He was admitted to the CCU for an NSTEMI, and you are trending troponin to peak. You are called to bedside because the patient is having increased chest pain. You obtain a repeat ECG showing ST-elevations in the anterolateral leads. You notify the overnight cardiology fellow who activates the cath lab for an emergent coronary angiogram and probable PCI.
Ask Yourself:
1. If the patient developed hemodynamic instability between now and the procedure, what potential MI complications would you be concerned for and what tests would you do?
2. As the cath lab team mobilizes, what additional medical management should you think about for the patient?
3. After the patient returns from the cath lab and in the coming days and weeks following hopefully a successful PCI, what complications would this patient be at risk for (related to either the MI itself or the PCI)?
4. What follow-up does the patient need while in the hospital?
5. What follow-up does the patient need in the coming months?
Normal sinus rhythm with multiple ectopic beats with rates in the 90s. Right axis deviation but normal intervals. Notable ST elevations in V3-V6 with potential reciprocal depression in leads III and aVF
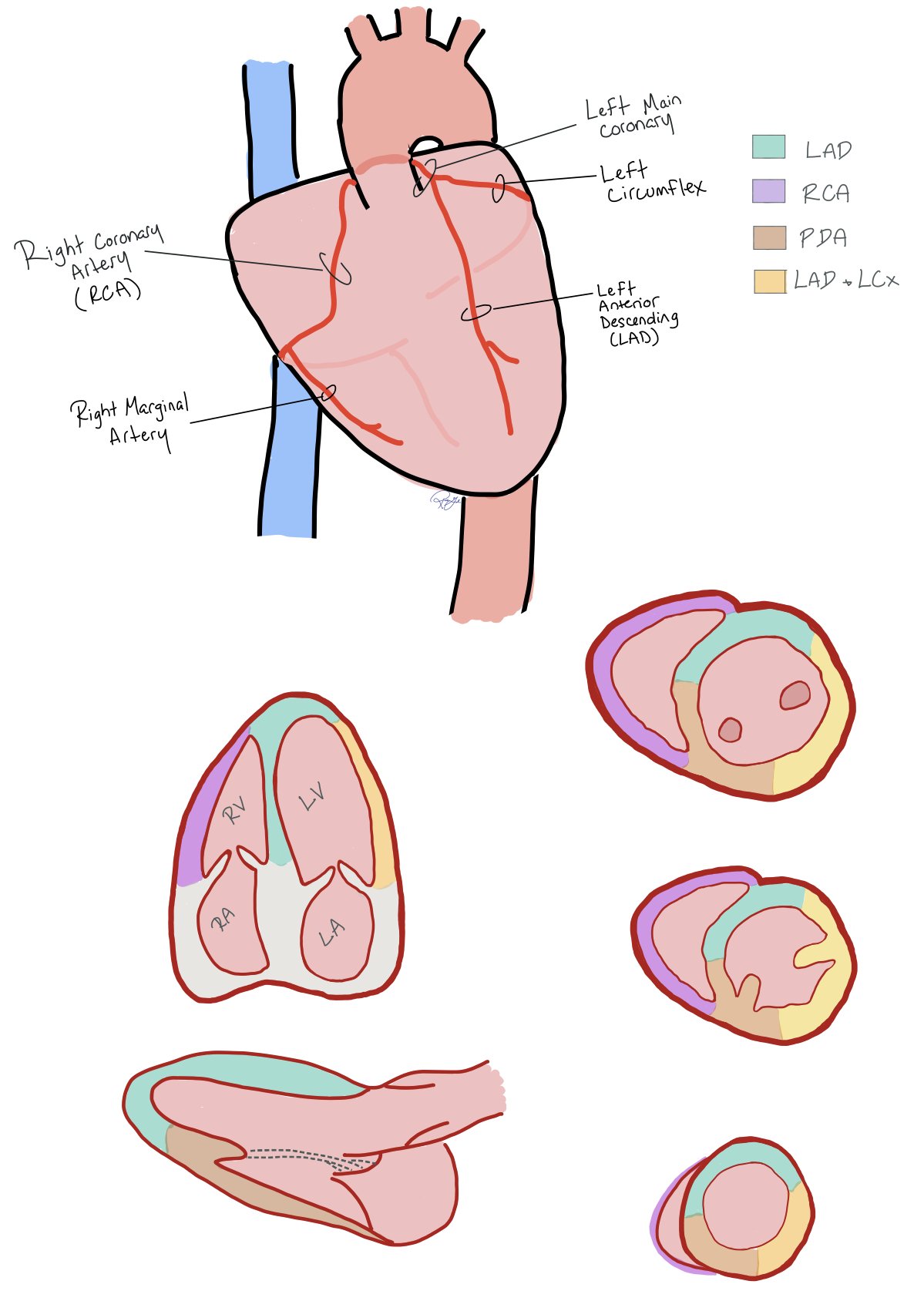
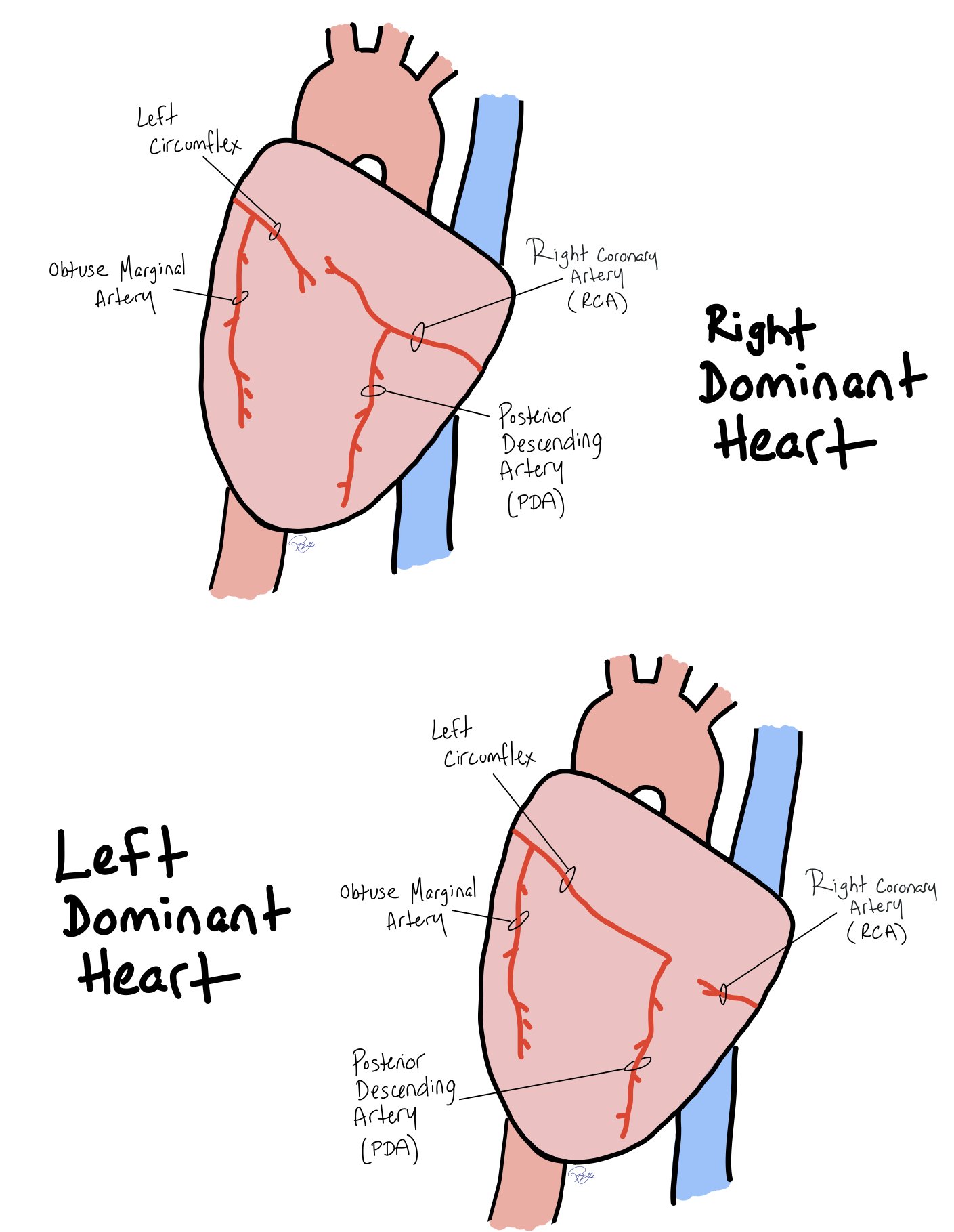
Coronary Distribution
This picture shows how coronary artery distribution to the back of the heart in both right and left dominant hearts.
This picture above shows the coronary artery distribution with correlation to POCUS view.
Background
We should have a few possible complications in mind as we approach care in the setting of acute MI. While incidence of these events has fallen significantly over time as intervention for acute MI has improved, we should remain aware of each complication. We’ll separate them into buckets: Mechanical, Pericardial, Thrombotic, and Electrical.
Mechanical Complications
Mechanical complications occur as a result of myocardial ischemia. They are rare events but may occur in patients who are not revascularized on time or who have large infarcts. When they do occur they are associated with high mortality.
Acute Mitral Regurgitation is a rare (incidence 0.05%-0.26%) but devastating (mortality 10-40%) complication secondary to papillary muscle rupture which occurs when corresponding coronary flow is compromised. The anterolateral papillary muscle branch is less commonly implicated as it has dual blood supply from the LAD and the diagonal or marginal branch of the LCx. The posteromedial papillary muscle on the other hand is more often involved as it has singular blood supply from the LCx or the RCA, depending on dominance. This will typically occur 3-5 days after transmural infarct, so you should think about this if the patient has had ongoing chest pain for days prior to presenting. On presentation there may be an MR murmur (which also may be absent due to rapid equalization of LA and LV pressures), pulmonary edema, or cardiogenic shock. Diagnosis is made with echocardiography. As the CCU resident, you can POCUS the patient to evaluate for this: evaluate the mitral valve in the parasternal long axis view and the apical 4 chamber view both in 2D and with color doppler over the valve. Management will consist of treating the cardiogenic shock – specifically, ensuring the patient has good circulation and oxygen delivery to perfuse their organs. This can be achieved with vasopressors, inotropes, or supplemental oxygen / positive pressure ventilation. In some cases, mechanical support can be used to help increase systemic circulation and decrease myocardial oxygen demand. The definitive management for acute mitral regurgitation is to fix the valve, whether it be through mitral clip or mitral valve surgery. The sooner these interventions can happen, the better.
Ventricular Septal Defect is another rare (incidence 0.3%) but serious (morality 30%-40%) event. Anterior and apical VSDs are due to LAD infarcts. Posterior VSDs are due to inferior (PDA) infarcts. They will typically occur 3-5 days after transmural infarct. Clinical features include dyspnea, orthopnea, a pansystolic murmur commonly at lower left sternal border, pulmonary edema, and cardiogenic shock. Diagnosis will be made with echocardiography. Think you hear a classic murmur when you are admitting the patient? POCUS them in subcostal, apical 2-, 3-, and 4- chamber views with color doppler over the septum. Management includes immediate afterload reduction with IABP or other mechanical circulatory support. Eventually surgical repair is needed, and transcatheter repair may be considered in poor surgical candidates.
Free Wall Rupture is the most common (incidence 2%) complication of acute MI but typically presents as an out of hospital sudden cardiac death. It is caused by transmural infarction of the myocardial wall. As with acute MR and VSD, it will present 3-5 days after transmural infarction. Clinical features include chest pain, JVD, pericardial effusion or cardiac tamponade (pulsus paradoxus, muffled heart sounds) and sometimes hemodynamic collapse. ECG may show electromechanical dissociation or ST elevations. Echocardiography will reveal the diagnosis. POCUS this patient to evaluate for pericardial effusion and tamponade (parasternal long, short, subcostal, and apical views) – your bedside ultrasound can immediately impact care to stabilize a critically ill patient. This complication warrants emergent surgical correction. Even if surgical repair is achieved, in-hospital mortality exceeds 35%.
LV Pseudoaneurysm is rare (0.2-0.3%) and occurs when cardiac rupture is contained by pericardial adhesions or scar tissue. Timing is variable, 3-80 days from infarction. It may be asymptomatic, although the majority of cases will present with signs and symptoms of heart failure, chest pain, and or dyspnea. Patients may have a systolic or diastolic murmur. ECG changes can be seen which may include ST elevations or be nonspecific. Diagnosis often requires use of multiple tools including TTE, TEE, Cardiac MR, Cardiac CT, Coronary angiography, and contrast ventriculography. Emergent surgical management is indicated as this presents high risk of rupture.
Picture above showing characteristics of mechanical complications of acute MI.
True LV Aneurysm most commonly involves a total thrombotic occlusion of the LAD which leads to a thin, scarred, or fibrotic myocardial wall which out pouches into an aneurysm. Most commonly it involves the anterior or apical walls of the LV. Often it contains organized thrombus – if you see an aneurysm without thrombus, preventive anticoagulation should be considered. LV Aneurysm may occur from 48 hours to 2 weeks post infarction. Clinical features include chest pain, signs and symptoms of heart failure, or ventricular arrhythmias. TTE with echo contrast will identify most cases, but cardiac MR is the gold standard for diagnosis. Management typically is conservative. You will need to determine if an LV thrombus is present and provide appropriate anticoagulation. Aneurysmectomy may be performed in some cases.
Cardiogenic Shock can be a result of any of the above mechanical complications or may be due to poor LV function in the setting of a large MI.
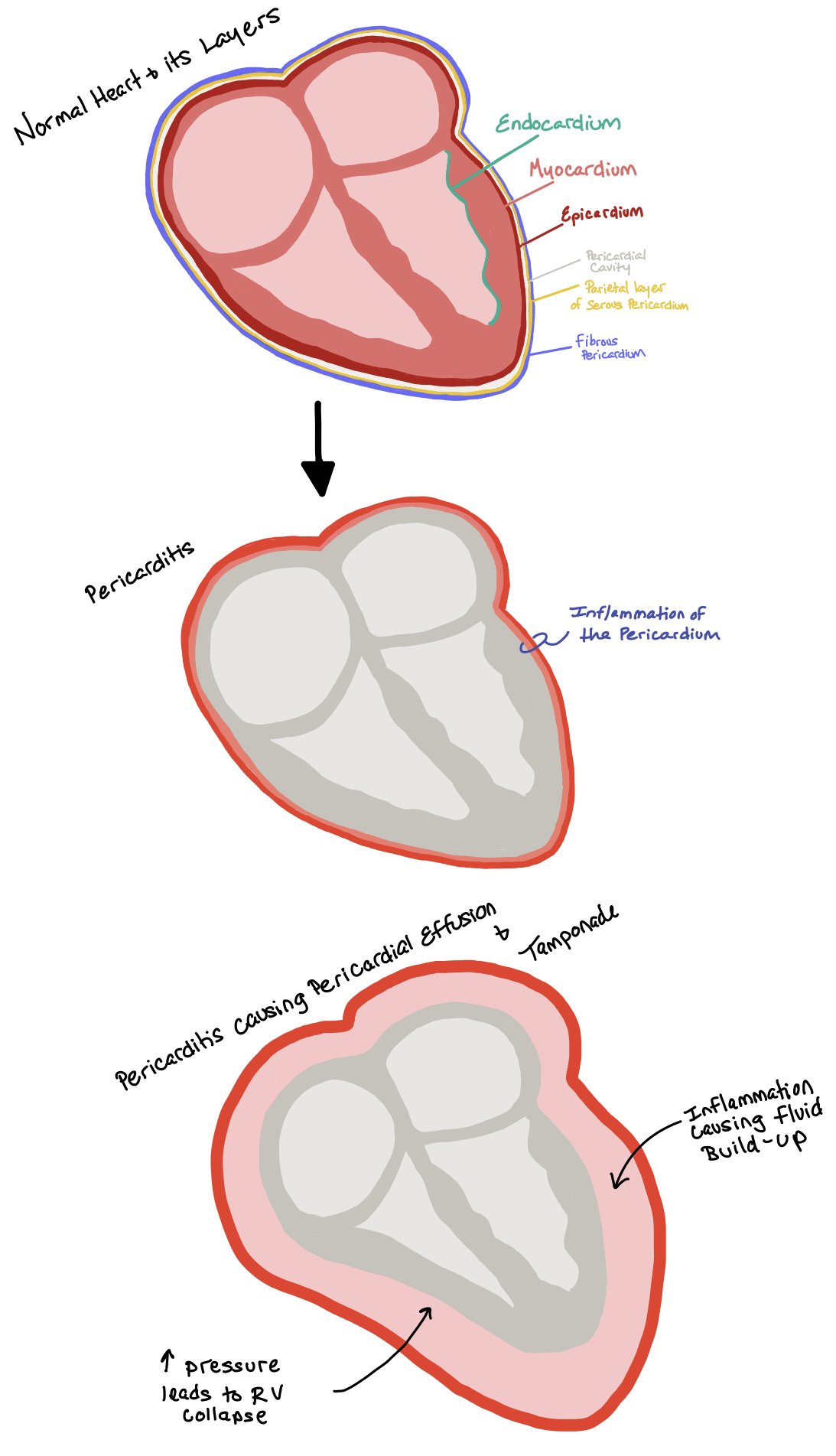
Pericardial Complications
These complications each involve the pericardial sac and are also related to the location and severity of myocardial injury.
Peri-Infarction Pericarditis is rare (incidence 1.2-5%) and caused by inflammation after transmural infarction. It occurs soon after MI, typically within 1-2 days. It presents with pericardial friction rub on exam, central pleuritic chest pain that improves with leaning forward and trapezius pain. On ECG, diffuse ST elevations or PR depressions as in other causes of pericarditis may be present. Diagnosis is typically clinical – Pericarditis diagnosis is made with at least 2 of the following criteria: classic chest pain, pericardial friction rub, classic ECG changes, (STE PR depression), and pericardial effusion. These patients should have a TTE which may reveal a pericardial effusion mostly to evaluate for other post-MI complications. Treatment with colchicine is first-line, with consideration of high-dose ASA or steroids if needed for pain relief. It is not associated with significant complications such as tamponade or high mortality.
Post-MI Pericardial Effusion may occur in patients with acute STEMI. Generally, it is caused by inflammation after transmural infarction, however you must be aware of the possibility of free wall rupture or coronary artery perforation (post-PCI) for large, acute, or hemodynamically-significant pericardial effusion causing cardiac tamponade. Outside of these acute diagnoses, trivial or small pericardial effusions after MI are often asymptomatic. TTE will reveal the diagnosis. Most often no treatment is indicated for small effusions. If there is tamponade physiology, pericardiocentesis should be performed emergently. If the patient meets clinical criteria for pericarditis, they should be treated accordingly.
Post-MI (Dressler) Syndrome is a pericarditis-like phenomena whose incidence has been greatly reduced in era of early reperfusion, and may be as low as 0.1%. Myocardial injury triggers an immune-mediated response. Typically, symptoms present weeks to months post-MI. Diagnosis will be made using a combination of clinical history, ECG, physical exam, and echocardiogram findings. The treatment is NSAIDs +/- colchicine with systemic glucocorticoids for refractory cases. It is not dangerous by itself, but does pose a risk of development of constrictive pericarditis if not treated appropriately.
Thrombotic Complications
Left Ventricular Thrombus occurs in around 4-8% of acute MI patients in the reperfusion era. In these cases, large and typically anterior MIs generate large areas of akinetic myocardium leading to blood stasis and thrombus formation. Most occur within the first 2 weeks post-MI and can occur within 24h. Most commonly the diagnosis will be made with TTE with echo contrast, but CMR is the gold standard. Anticoagulation should be ordered for prevention of stroke and systemic embolization.
Another thrombotic complication is “Plaque Shift,” where some of the thrombus from the acute occlusion MI can shift downstream and cause occlusion of distal or side branches. When this happens, the occlusion can even block a large territory (ex. a large diagonal or obtuse marginal branch). This occurs at the same time as the PCI, but may only be recognized hours later when the ECG changes are noticed or the patient has persistent angina despite the open index culprit vessel being open. If a patient has a PCI with post-intervention ECG showing improvement in ST deviations but then starts developing chest pain and dynamic ECG changes, they may have distal occlusion due to plaque shift and may need to go back to the cath lab for repeat coronary angiography. Medical management may include addition of a Gp2b3a inhibitor to dissolve thrombus in addition to nitroglycerin (SL or gtt) for chest pain relief.
If patients do not take their DAPT as prescribed, another fatal complication is Stent Thrombosis in which the stent itself becomes acutely occluded (think Stent Thrombosis showing acute ST elevation MI). Remember not to confuse Stent Thrombosis with In-Stent-Restenosis, which is a more chronic process and why we use drug eluding stents (DES) to help prevent epithelization and stent blockage over time.
The picture above shows how an LV thrombus is formed. When a patient has an MI (specifically an LAD infarct), stasis occurs. Together, this leads to hypercoagulability and LV thrombus formation.
Electrical Complications
Conduction Abnormalities: Conduction abnormalities following MI are a result of reperfusion injury, autonomic irregularity, or infarction of the conduction system itself. The location of the infarct may influence what type of conduction issue may arise. They occur between 1 and 3% of the time. They may be present on admission for acute MI or develop later in the hospitalization.
For an inferior MI: The RCA commonly supplies the SA node, AV node and His bundle. As such, Sinus Bradycardia, 1st degree AV block, 2nd degree Mobitz Type 1 AV block and Complete Heart Block may be seen with RCA occlusion. Stable patients with narrow escape rhythms may be monitored. Typically these abnormalities will be transient, resolving within 5-7 days. Unstable or symptomatic patients should be treated with atropine and may require temporary subcutaneous or transvenous pacing.
Blood supply of the cardiac conduction system, curtesy of ecgwaves.com
Additionally, when patients have acute inferioroposterior myocardial infarctions, they can develop bradycardia, hypotension, and apnea-- aka the Bezhold-Jarsich Reaction. This occurs due to inhibitory mechanoreceptors in the left ventricle that inhibit sympathetic activity when the left ventricle is poorly filled.
For an anterior MI: Right and left bundle branch blocks may be seen in LAD infarction. It is uncommon to see 1st degree AV block. 2nd degree Mobitz type II block may occur, typically at or below the level of the His bundle. Complete heart block can develop as well. Generally these conduction disturbances are expected within the first 24 hours. These patients generally will not respond to atropine and will require temporary (and often permanent) pacing due to the large extent of infarction required to cause these disturbances.
Supraventricular Arrhythmias may also develop in the setting of acute MI, including Atrial Fibrillation, Atrial Flutter and Paroxysmal supraventricular tachycardia. There may also be sinus tachycardia in the setting of poor LV function. SVTs should be managed in the same way as for non-MI related SVT (Click here to learn more about SVT). The important point here is not to miss an acute MI if a patient presents with chest pain and SVT - troponin trends and LV wall motion (by POCUS or TTE) are key if ST deviations are not obvious.
Ventricular Arrhythmias are the most common cause of sudden cardiac death in the setting of acute MI. Types include Ventricular Tachycardia and Ventricular Fibrillation. They occur as a result of ongoing ischemia, catecholamine surge, myocardial scar formation, and electrolyte disturbances. Patients who develop these unstable arrhythmias have a 20% or greater in-hospital mortality. They develop mostly within the first 48 hours of an acute MI and may develop within the first 30 minutes of myocardial ischemia. If a patient has VT (even non-sustained) during or after PCI, use of lidocaine infusion may help to quiet arrhythmias and avoid cardiac arrest as the myocardium recovers. Lidocaine levels should be checked daily to ensure the level remains in the therapeutic range. Patients presenting with out-of-hospital cardiac arrest from VT or VF should be considered for emergent coronary angiography given the high probability of acute MI as the culprit.
Note: patients can have reperfusion ventricular arrythmias that, unlike ventricular tachycardia and ventricular fibrillation, is usually not associated with hemodynamic compromise!
Back to the Case:
1. If the patient were to develop hemodynamic instability between now and the procedure, what potential MI complications would you be concerned for and what workup would you do at the bedside?
Given that our patient’s chest pain began 3 days ago, we can assume that he’s been having myocardial ischemia since then. We can also expect that he is having an anterior (left main, LAD) and/or lateral (LCx) MI given his ECG findings. These two pieces of information put him at risk of having many of the beforementioned complications, including all of our mechanical complications (acute MR, VSD, Free Wall Rupture, and LV Pseudoaneurysm and Aneurysm), all of which may be causing this presentation. Though our patient could be suffering from Peri-Infarction Pericarditis given the timing, this is not likely to be causing hemodynamic instability, and the presence of regional (as opposed to diffuse) ST elevations also makes this unlikely. Our patient also may be developing garden variety cardiogenic shock in the setting of a large area of infarcted myocardium.
If you wanted to grab the POCUS, you would be correct! We should also have this patient on continuous telemetry as any of our electrical complications could be occurring in this timeframe. We also want to do a good physical exam looking for signs of fluid overload as well as good cardiac auscultation, looking for new murmurs.
2. As the cath lab team mobilizes, what additional medical management should you think about for the patient?
The name of the game is no chest pain! If hemodynamically stable, you can give sublingual nitroglycerin (or a drip if the sublingual x3 doses is not enough) in order to ensure the patient is not having any chest pain, as pain increases the patient’s sympathetic response and, thus, increases HR, BP, and oxygen demand. Morphine and fentanyl should be used very sparingly. Ensure the patient’s O2 > 90% -- otherwise, no need for additional oxygen as it has been shown to cause more harm than good. In ACS, order heparin (inhibit the coagulation cascade), aspirin (inhibit the platelet activation), and high dose statin (stabilize the lipid core). You can take with the fellow about adding a P2Y12 inhibitor vs waiting for it to be done in the cath lab as many times we choose P2Y12 inhibitors based on lesion type. Additionally, if the patient requires a CABG, many CT surgeons will not operate with a P2Y12 inhibitor in the system – as such, we sometimes wait to see the coronary anatomy before loading the patient with a P2Y12 inhibitor in case they need an immediate CABG after the PCI.
If the patient has a contrast allergy, you will need to pre-treat with steroid and Benadryl!
If there is still time, you can POCUs the patient to see if there are any new wall motion abnormalities or mechanical complications as this may help the team and the cath lab decide if the patient needs a RHC/Swan, mechanical circulatory support, or surgical consultation.
Given that many these patients are critically ill and may require emergent surgery, consider having an active type and screen!!
3. After the patient returns from the cath lab and in the coming days and weeks following hopefully a successful PCI, what complications would this patient be at risk for (related to either the MI itself or the PCI)?
Our patient will remain at risk for most mechanical complications, with decreasing risk after day 5 (2 days from now) for MR, VSD and FWR. He will remain at risk for LV Pseudoaneurysm and true Aneurysm for multiple weeks. A post-MI pericardial effusion may be identified for weeks after the procedure as well but will be unlikely to cause problems. Dressler syndrome may cause pericarditis-like symptoms for months post-MI. An LV thrombus may develop in the coming days, most often within 2 weeks. This patient will remain at risk for arrhythmias related to acute ischemia and (later) scar formation, especially in the setting of late-presenting MI with a large infarcted territory. Due to his likely very significant MI, he falls into a high risk group for all complications.
The patient will be at risk for post-PCI complications, most importantly stent thrombosis, which can occur acutely or as late as one year in the future. The importance of taking DAPT should be impressed upon the patient by all members of the care team.
4. What follow-up does the patient need while in the hospital?
A repeat ECG should be obtained immediately after PCI to evaluate for improvement of ST deviations. If the patient develops more chest pain later, this new baseline ECG will be needed as a comparison. A complete echo should be obtained before discharge in order to determine need for future HFrEF therapies and presence of LV thrombus or LV aneurysm. LVEF may improve days to months after acute MI. Of course, we should also provide appropriate post-MI medical management and lifestyle counseling. Smoking cessation counseling is an essential part of this and it is absolutely necessary to provide this during a patient’s index presentation with acute MI.
5. What follow-up does the patient need in the coming months?
Patients who have new or worsening heart failure should be optimized on GDMT with plan for repeat complete TTE 40 or more days post-MI. CD therapy and HFrEF GDMT considered if LVEF is still low and the patient is continuously symptomatic.This can be done as part of a cardiac rehab program, which is recommended for all post-MI patients.
All patients should have their lipids reassessed 4-8 weeks after MI! See the “Lipid Chapter” for more specific details!
Further Learning:
Resident Responsibilities
Be aware of these possible complications, especially if your patient is having new symptoms or is hemodynamically unstable. Keep these in mind during any RRT for a patient with ACS or post-MI. POCUS can be very useful in these situations.
Order a complete TTE before your patient discharges.
Order a repeat ECG immediately after PCI. Daily ECGs should also be ordered until discharge.
For post-MI care, remember to order daily P2Y12 inhibitor and aspirin! All patients with MI should have high-intensity statins with plan for repeat follow up of lipid levels 4-8 weeks after discharge (aiming for >50 reduction). Consider adding ACEi/ARB/ARNIs and beta blockers in all patients prior to discharge (ARNI preferred in reduced EF).
Make sure to refer these patients to Cardiac Rehab!! Also, all patients post-MI should have scheduled follow up with their cardiologist prior to discharge!!
Don’t forget to give the influenza vaccine to all patients prior to discharge!
Further Reading:
How’d we do?
The following individuals contributed to this topic: Drew Carlson, MD; Catherine Vanchiere, MD; Amer Ardati, MD
Chapter Resources
O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127(4):362. doi:10.1161/CIR.0b013e3182742cf6
Overview of the nonacute management of ST-elevation myocardial infarction - UpToDate. . https://www.uptodate.com/contents/overview-of-the-nonacute-management-of-st-elevation-myocardial-infarction?search=stemi%20manegemtn&source=search_result&selectedTitle=2%7E150&usage_type=default&display_rank=2#H41.
Periprocedural complications of percutaneous coronary intervention - UpToDate. . https://www.uptodate.com/contents/periprocedural-complications-of-percutaneous-coronary-intervention?search=PCI%20omplications&source=search_result&selectedTitle=1%7E150&usage_type=default&display_rank=1#H24.
Ventricular arrhythmias during acute myocardial infarction: Incidence, mechanisms, and clinical features - UpToDate. . https://www.uptodate.com/contents/ventricular-arrhythmias-during-acute-myocardial-infarction-incidence-mechanisms-and-clinical-features?search=mypocardial%20infarction%20arrhythmias&source=search_result&selectedTitle=1%7E150&usage_type=default&display_rank=1#H11423244.
Supraventricular arrhythmias after myocardial infarction - UpToDate. . https://www.uptodate.com/contents/supraventricular-arrhythmias-after-myocardial-infarction?search=mypocardial%20infarction%20arrhythmias&source=search_result&selectedTitle=3%7E150&usage_type=default&display_rank=3#H701771643.
Conduction abnormalities after myocardial infarction - UpToDate. . https://www.uptodate.com/contents/conduction-abnormalities-after-myocardial-infarction?topicRef=63&source=see_link.
Anton C, Valentin F, Gennaro G, et al. Left Ventricular Thrombus Following Acute Myocardial Infarction. J.Am.Coll.Cardiol. 2022;79(10):1010-1022. doi:10.1016/j.jacc.2022.01.011
Left ventricular thrombus after acute myocardial infarction - UpToDate. . https://www.uptodate.com/contents/left-ventricular-thrombus-after-acute-myocardial-infarction?topicRef=63&source=see_link.
Pericardial complications of myocardial infarction - UpToDate. . https://www.uptodate.com/contents/pericardial-complications-of-myocardial-infarction?topicRef=63&source=see_link#H187797.
Sattar Y, Alraies MC. Ventricular Aneurysm. In: Ventricular Aneurysm. In: StatPearlsStatPearls Publishing; 2024. http://www.ncbi.nlm.nih.gov/books/NBK555955/.
Csapo K, Voith L, Szuk T, Edes I, Kereiakes DJ. Postinfarction Left Ventricular Pseudoaneurysm. Clin Cardiol. 2009;20(10):898-903. doi:10.1002/clc.4960201021
Left ventricular aneurysm and pseudoaneurysm following acute myocardial infarction - UpToDate. . https://www.uptodate.com/contents/left-ventricular-aneurysm-and-pseudoaneurysm-following-acute-myocardial-infarction?topicRef=63&source=see_link.
Acute myocardial infarction: Mechanical complications - UpToDate. . https://www.uptodate.com/contents/acute-myocardial-infarction-mechanical-complications.
Abdulla A. Damluji, MD, PhD, MPH, FAHA, Chair, Sean van Diepen, MD, MSc, FAHA, Vice Chair, Jason N. Katz, MD, MHS, FAHA, Venu Menon, MD, FAHA, Jacqueline E. Tamis-Holland, MD, FAHA, Marie Bakitas, DNSc, CRNP, Mauricio G. Cohen, MD, FAHA, Leora B. Balsam, MD, and Joanna Chikwe, MD. Mechanical Complications of Acute Myocardial Infarction: A Scientific Statement From the American Heart Association. 2021. doi:10.1161/CIR.0000000000000985
Kaul S, Methner C, Cao Z, Mishra A. Mechanisms of the “No-Reflow” Phenomenon After Acute Myocardial Infarction: Potential Role of Pericytes. JACC Basic to translational science. 2023;8(2):204-220. doi:10.1016/j.jacbts.2022.06.008