Case Presentation:
A 68-year-old male with a past medical history of hypertension (HTN), hyperlipidemia (HLD), type 2 diabetes, and tobacco use disorder presents with one week of new-onset, progressive exertional dyspnea, and lower extremity edema. He started having difficulty sleeping in his usual position and wakes up frequently at night due to this shortness of breath. Prior to the onset of his symptoms, he was able to walk several blocks without issue but now becomes short of breath with minimal exertion.
Vitals: BP 90/70, HR 90 bmp, RR 22, O2 sat: 90% on 2L O2
Physical Exam
General: Sitting upright in bed, appears uncomfortable with labored breathing.
Cardiac: Regular rate and rhythm, JVD 12 cm, + hepatojugular reflux
Pulm: bilateral rales
MSK: 2+ pitting edema bilaterally to knees
Labs
CBC: wnl; BMP: Cr 1.7 (baseline 0.9). Electrolytes wnl; BNP: 1600 pg/ml; High Sensitivity Troponin-I: 15 -> 19
EKG: Compared to prior baseline EKG 6 months prior. Deep Q wave seen in leads V1, V2, V3, which were not present previously. Otherwise sinus rhythm, rate in the 90s
CXR: pulmonary vascular congestion, cardiomegaly
TTE: LVEF 30%, moderate global hypokinesis, anterior wall motion abnormality
Ask Yourself:
What is the differential diagnosis? What factors support one diagnosis as the most likely?
How is heart failure classified and why does it matter?
How do we approach diuresis and volume management in this patient?
How do we initiate and titrate GDMT in a patient with newly diagnosed HFrEF? What additional medications and advanced therapies can be used for symptom management in heart failure?
Diagnosing Heart Failure
Differential Diagnosis
Dyspnea and peripheral edema are some of the most prominent clinical signs and symptoms of acute decompensated heart failure (ADHF) exacerbations. Depending on the specific situation, the differential may include (but is not limited to):
● Cardiac pathologies, including various HFrEF, HFpEF, valvular, pericardial effusions / tamponade / constriction, arrythmias, pulmonary HTN, cardiomyopathies, valvular heart disease, and pericardial disease)
● Pulmonary diseases (obstructive and restrictive lung disease, pulmonary embolism, and pulmonary hypertension)
● Nephrotic syndrome
● Cirrhosis
When differentiating between these causes, it is important to consider context, such as the patient’s medical history and symptom onset which can point toward certain etiologies.
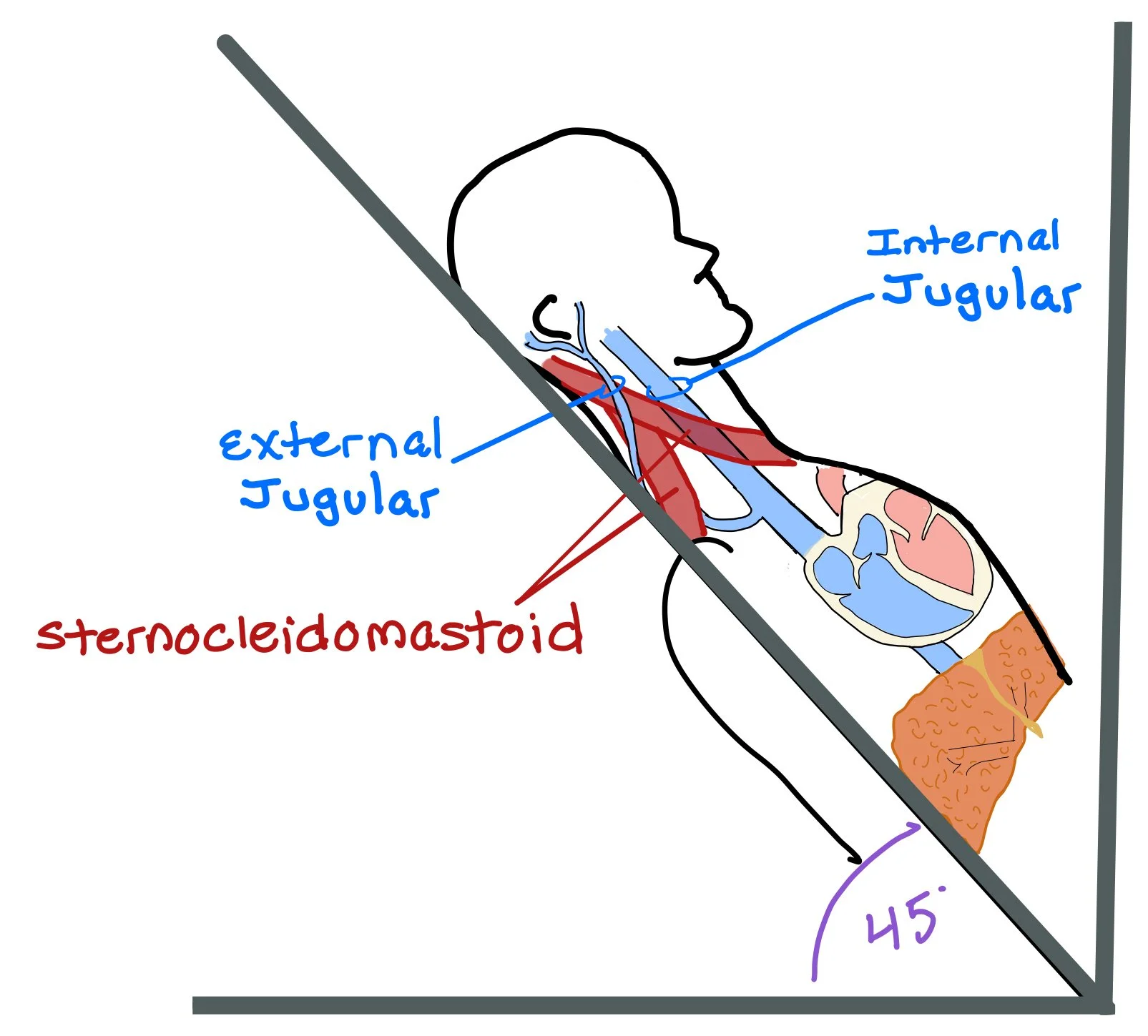
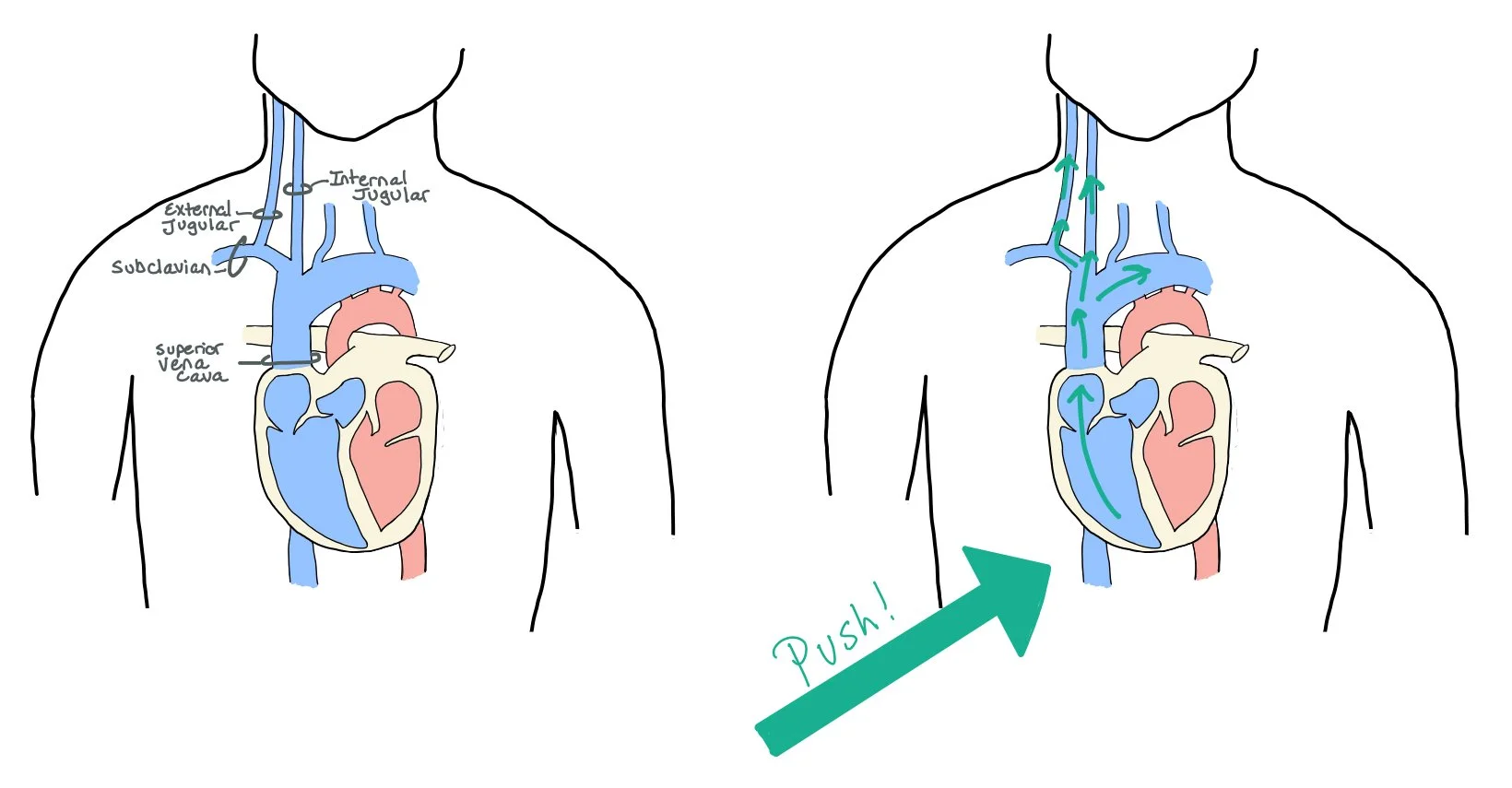
Since the right internal jugular (IJ) sits directly on top of the superior vena cava (SVC) and right atrium of the heart, pushing on the abdomen and looking at the IJ allows us to see the jugular-venous distention (JVD) and get a better look at the right atrial pressure and whether or not the patient is fluid overloaded.
When assessing for JVD, we lay the patient back at a 45 degree angle. Usually, we expect the pulsation of the IJ to be ~3 cm over the sternal angle. The patient will then turn their head towards their left shoulder, which will help open the space between the sternocleidomastoid muscles and allow us to see the IJ.
How do we know if we are looking at the IJ and NOT the carotid?
Take the patient’s radial pulse. If what you’re looking at beats with the pulse, you are looking at the carotid! For the IJ, you will expect two pulsations for every heart beat.
The JVP is usually not palpable! If you can feel it pulsating, you may be feeling the carotid!
Look for venous engorgement. If you lightly hold your finger at the base of the IJ, you will start to see venous engorgement of the vein!
Assess for the hepatojugular reflex! Pushing on the abdomen underneath the liver will cause increased venous engorgement and back pressure up the IJ. By doing this test, you can hopefully now see where the patient’s baseline JVD lays.
Heart failure is a clinical diagnosis!!! The diagnosis is made based on the presence of clinical features that are due to structural or functional cardiac dysfunction. This diagnosis can be supported with:
Labs:
Elevated brain natriuretic peptide. BNP <100 pg/mL provides strong negative predictive value for heart failure exacerbation (although BNP is not specific to HF and may be due to afib, tamponade, PE, etc)
Additional laboratory tests for all patients diagnosed with heart failure should include CBC, UA, BMP, lipid profile, serum glucose, LFTs (look at the albumin as low albumin can cause edema!), iron studies, and TSH to optimize management
TTE: preferred imaging modality that allows for assessment of systolic, diastolic, and valvular function, which may guide you towards identifying an underlying cause. Regional wall motion abnormalities can be suggestive of ischemic disease
CXR: often demonstrates cardiomegaly and pulmonary vascular congestion
ECG: can often be normal, but may provide insight into underlying etiology or comorbid cardiac pathologies including ischemic events
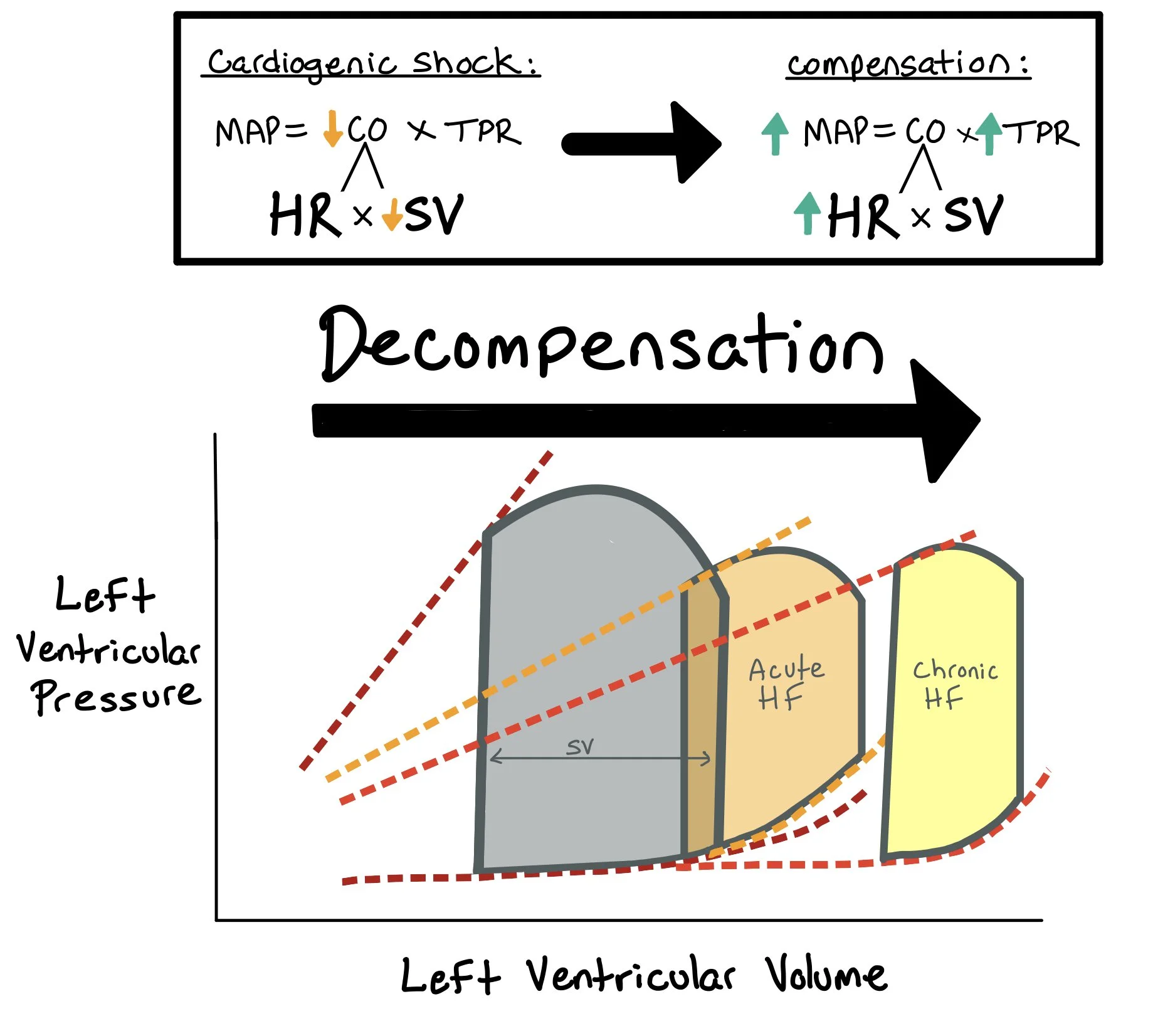
When evaluating patients with heart failure, it’s important to determine if they are appropriately perfusing their organs or if they are in shock. When patients have heart failure, the heart works less efficiently and their cardiac output (CO) decreases. The body tries to compensate by increasing the total peripheral resistance to maintain the mean arterial pressure (MAP = CO x TPR). If the body cannot compensate for the decreased cardiac output, the MAP will decrease, and the patient will not be able to perfuse their vital organs.
When patients have acute decompensated heart failure, the mean arterial pressure (MAP) decreases because stroke volume (SV) decreases. As a compensatory response, the body will increase the systemic vascular resistance (SVR) to help protect the MAP as low MAPs lead to poor perfusion and organ damage.
** Note: A normal MAP (>65) does not exclude cardiogenic shock!!! This would be categorized as normotensive cardiogenic shock. One should look at pulse pressure and proportional pulse pressure -- both of which are surrogates for stroke volume and help identify this “normotensive” cardiogenic shock.
A Swan Ganz Catheter is often critical to determine if cardiogenic shock is present, assess the degree of left heart and right heart involvement, and aid in treatment decisions. This allows for assessment of cardiac filling pressures, cardiac output, and systemic and pulmonary vascular resistances. A Swan Ganz catheter importantly allows for measurement of pulmonary artery pressures and the pulmonary capillary wedge pressure (PCWP), a surrogate for left atrial pressure. Low cardiac output/index and high filling pressures are characteristic of cardiogenic shock. When a Swan Ganz catheter is unavailable, a central line from the internal jugular vein can provide adequate diagnostic information by obtaining a central venous pressure (CVP) measurement and central venous oxygen saturation (CVO2), which can be used to calculate cardiac output and index.
Note: When a patient is in cardiogenic shock, they have decreased CO due to the decreased SV. In these cases, the patient may be dependent on HR to help compensate for the low SV and to maintain the CO. That said, we must be VERY careful when giving medications that decrease the HR as this can lead to decompensation if not done appropriately.
Heart Failure - A Clinical Diagnosis
Heart failure may present with fatigue, exertional dyspnea, and exercise intolerance. Other classic signs and symptoms and physical exam findings of HF can be differentiated by “left-sided” and “right-sided” features.
Left-sided features are a result of pulmonary congestion due to failure of the left side of the heart causing back-up of fluid into the lungs.
Typical symptoms include:
○ Progressive dyspnea (first with exertion but eventually seen at rest)
○ Orthopnea (shortness of breath upon lying down that improves with sitting up)
○ Paroxysmal nocturnal dyspnea (nighttime episodes of acute shortness of breath)
○ Cardiac asthma (bronchospasm that occurs due to increased pressure in bronchial arteries that may mimic asthma)
○ Exam findings can include bilateral basilar rales, but these can also be absent in chronic disease – don’t be falsely reassured by the absence of rales.
Right-sided features are a result of increased central venous pressure (CVP) leading to systemic fluid retention.
Typical symptoms include:
○ Peripheral edema: often but not always seen as lower extremity edema or edema in dependent areas. Some patients may store fluid in less clearly visible body cavities
○ Hepatic venous congestion can result in abdominal pain and jaundice due to stretching of the liver capsule
○ Physical exam findings include jugular venous distention due to venous congestion from increased CVP and hepatojugular reflux.
** The most common cause of right-sided heart failure is left-sided heart failure, so it is possible to see physical exam findings consistent with both sets of features.
Staging vs. Functional Status
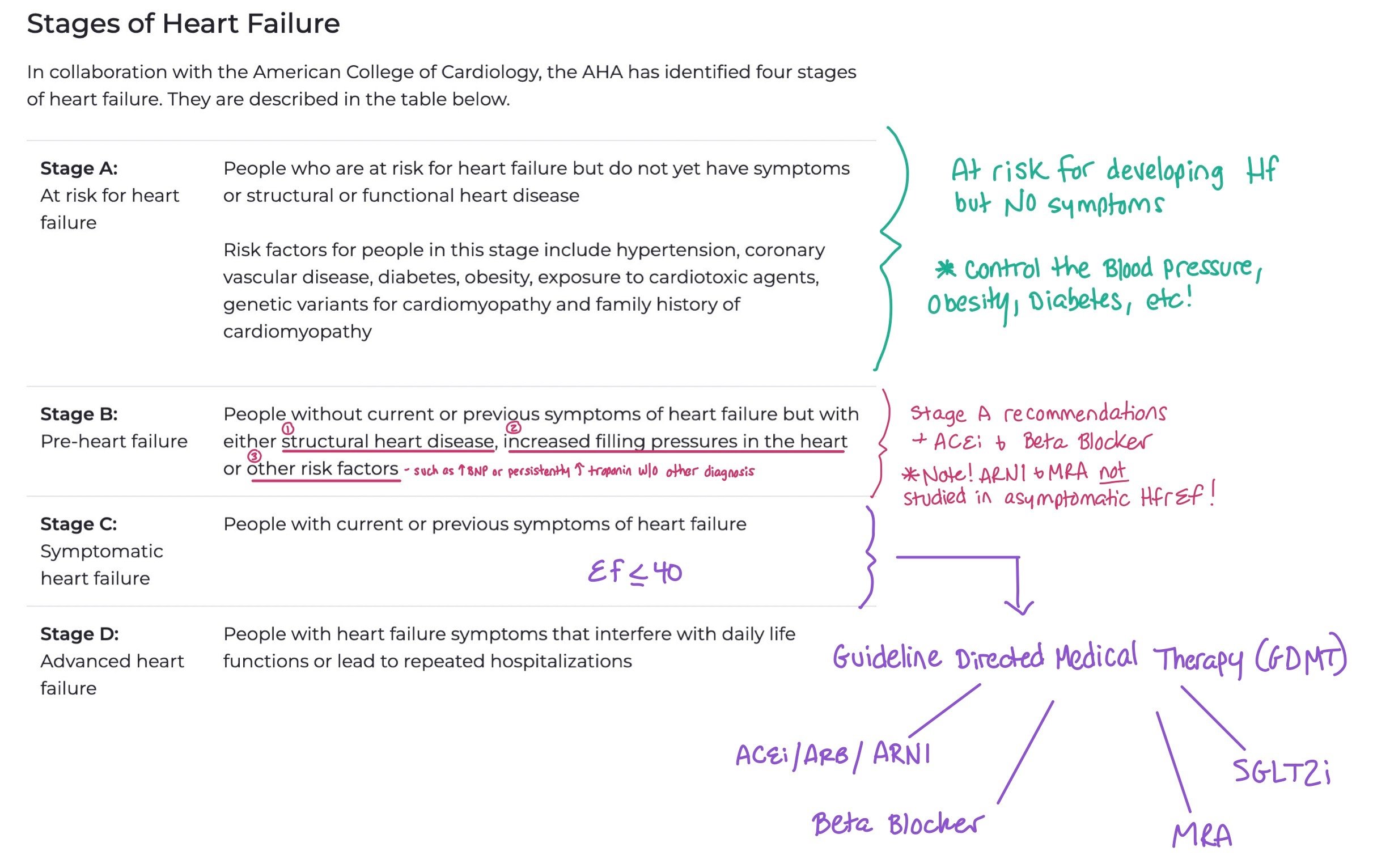
When we talk about heart failure, we typically refer to both the stage of the heart failure and the functional status, (aka. the New York Heart Association (NYHA) Functional Classification). Knowing the staging allows providers to understand how advanced the heart failure is, while providing the functional classification allows them to assess and treat patient symptoms. Staging refers to progression of heart failure, while function refers to symptoms.
This picture breaks down the different stages of heart failure. When patients have Stage A, the main intervention is trying to control their risk factors for developing heart failure, such as controlling their blood pressure, diabetes, and obesity. For Stage B, you can also add an ACEi and Beta Blockers. Finally, for Stage C and D with HFrEF < 40, this is when we start to add the GDMT.
Note: Unlike functional status that is constantly changing, stage progression only goes in one direction (i.e. once patients have Stage C they cannot go back to stage B).
The NYHA Functional Classification is based on the patient’s symptoms, which can fluctuate based on whether or not the patient is decompensated or not.
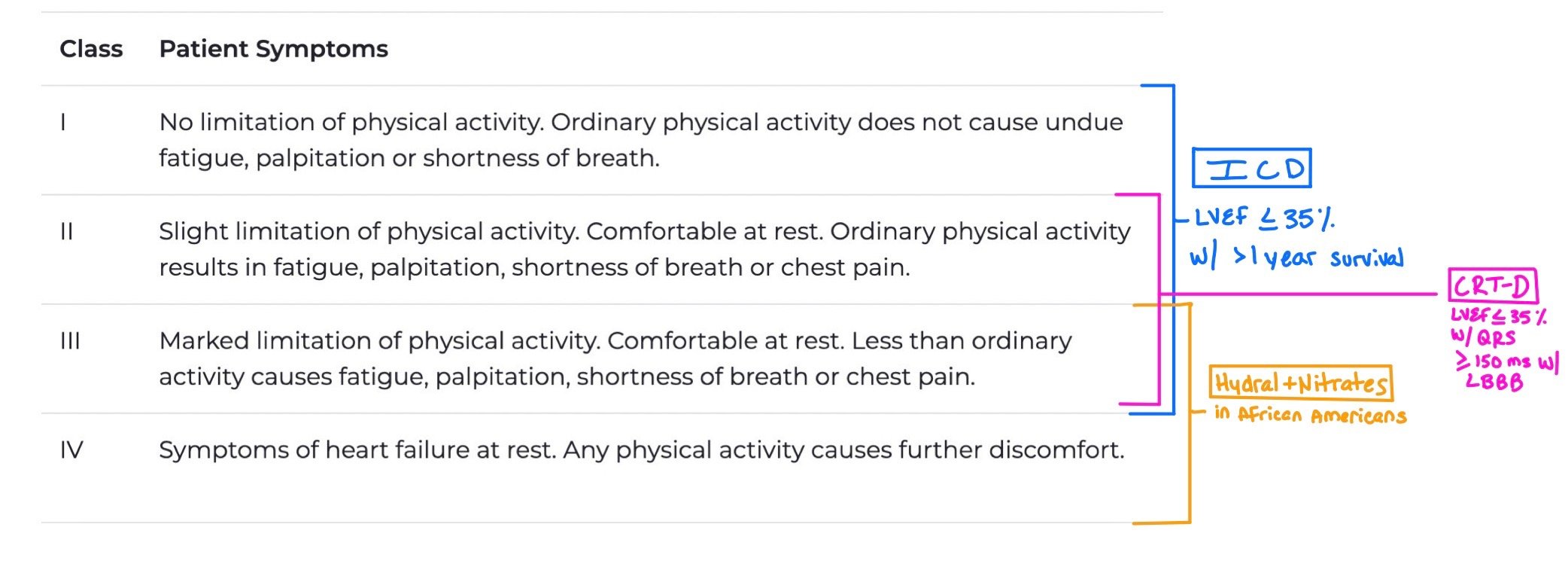
When patients have Class I-III with LVEF < 35% and greater than 1 year survival, they should get an ICD.
When patients have Class II-III with LVEF < 35% with QRS > 150 ms and left bundle branch block (LBBB), they are candidates for CRT-D.
Patients with Class III-IV who are African American should get hydralazine + nitrate
The following charts from the 2022 AHA/ACC/HFSA Heart Failure Guidelines help to demonstrate how we use both staging and functional class to treat our patients. The first chart shows how we go about treating patients with Stage A and B, while the second picture shows treatment for Stage C and D.
This figure shows treatment for Stages A and B. The green boxes denote Class 1 recommendations (strong evidence with high benefits) while yellow is Class 2a (moderate evidence). As patients in Stage A are classified at risk for developing heart failure, therapies are targeted to help them prevent progression by treating risk factors. For Stage B, we continue to try to prevent progression by treating underlying disease; however, when patients start to develop HFrEF (i.e. EF <40) these patients benefit from ARNIs and beta blockers. If they qualify for an ICD due to EF < 30% >40 days after MI, this is considered a Class 1 recommendation.
This figure shows how we treat our patients with Stages C and D. Remember, these patients have already become symptomatic from their heart failure. Therapies are targeted to help prevent further disease progression with guideline directed medical therapy (GDMT) as well as additional therapies depending on their NYHA functional class if they continue to be symptomatic despite the fact that they are on maximally tolerated GDMT.
What about our patients with HFpEF?
Until recently, we did not have good therapies to treat patients who have diastolic dysfunction, aka heart failure with preserved ejection fraction (HFpEF, EF >50%). As ~50% of our patients with heart failure have HFpEF, determining therapies for these patients became critical. While the mainstay of therapies is to treat underlying risk factors (like hypertension, diabetes, renal disease, etc), we try to help these patients with symptom management, specifically with diuretics. SGLT2i are given a Class 2a recommendation as they help to augment diuresis and have been shown to decrease hospitalizations and length of stay (EMPEROR-PRESERVE),
The following figure from the 2022 AHA/ACC/HFSA Heart Failure Guidelines demonstrates HFpEF treatment. Note how ARNI, MRA, and ARBs are class 2b (i.e. weak recommendations). While not first line, they can be used to help control HTN and potential further progression of the patient’s heart failure.
Etiologies of Heart Failure:
Heart failure etiologies are generally classified as ischemic or non-ischemic. First, we rule out ischemic causes of heart failure. If ischemia is ruled out, we look for non-ischemic causes.
Ischemic cardiomyopathy causes over 50% of cases of heart failure with reduced ejection fraction and is primarily caused by coronary artery disease and myocardial infarction, leading to reduced blood flow and damage to the heart muscle.
Suspect ischemic heart disease when ST or Q wave changes are present on an EKG, wall motion abnormalities on TTE, troponin elevation, or chest pain with exertion or rest. Patients may likely have risk factors including diabetes, hypertension, smoking history, or hyperlipidemia.
Ischemic evaluation can be done with multiple modalities, including stress imaging (echo or nuclear) or coronary angiography—either invasive (i.e. left heart catheterization with coronary angiography) or via CT—which can help determine the extent and significance of CAD. Most patients with new onset heart failure should have an ischemic evaluation, often invasive coronary angiography to rule out that as a potential cause.
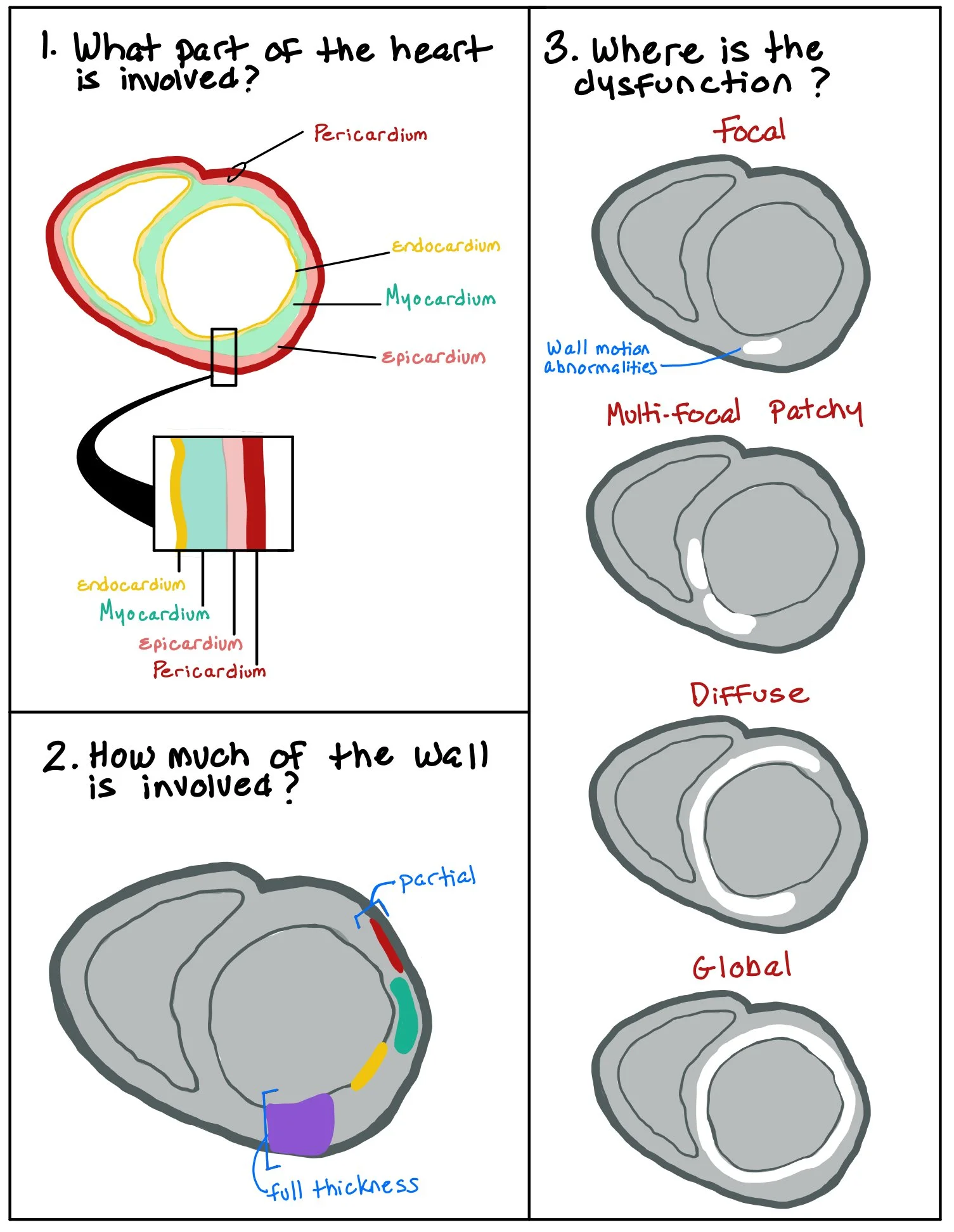
The picture above shows the different parts of the heart wall. When patients have wall motion abnormalities, we look to see how much of the wall thickness is involved (ex: normal, hypokinetic, dyskinetic, and akinetic), as well as whether the abnormality is focal or global.
Non-ischemic cardiomyopathy includes a wide range of causes, such as long-standing hypertension, valvular heart disease, tachyarrythmias, obesity, and diabetes. Other causes include genetic cardiomyopathies, infiltrative or inflammatory conditions, toxin exposure (e.g., chemotherapy or illicit substances), and endocrine or metabolic disorders. Identifying many of these causes requires a thorough history on presentation. This classification helps in guiding diagnostic workup and management strategies.
Cardiac MRI (CMR) is a noninvasive tool that can help identify heart failure etiologies by detecting myocardial fibrosis and scaring. When fibrosis and scaring are present, there will be different uptake and washout patterns of the gadolinium in the healthy vs abnormal cardiac tissue. Presence of late gadolinium enhancement (LGE) suggests scaring or necrosis. The specifc LGE pattern can suggest ischemic etiology (often subendocardial and distributed in a territory of a coronary artery) or some non-ischemic etiologies (for example, mid-myocardial LGE is often seen in non-ischemic or genetic cardiomyopathies, and patchy LGE in a non-coronary territory can suggest cardiac sarcoidosis). T1/T2 imaging and mapping techniques can suggest infiltrative or inflammatory cardiomyopathies and offer prognostic insights.
A Word About Cardiac Amyloidosis…
One major cause of heart failure, especially HFpEF, is Cardiac Amyloidosis.
Amyloid is a generic term for misfolded proteins that form beta pleated sheets. Since the body is unable to breakdown these proteins, the proteins will deposit into different parts of the body. Cardiac Amyloidosis, the most common type of restrictive cardiomyopathy, occurs when the amyloid deposits within the myocardium.
While there are many different types of proteins that can do this, we divide those proteins into two main groups:
AL Amyloidosis– This is a plasma cell disorder in which plasma cells make antibodies, which have body heavy and light chains. The light chains dissociate and come together to make the misfolded protein of amyloid. This is a toxic infiltrative disease as the amyloid deposits and causes myocyte apoptosis.
ATTR– Occurs when the transthyretin protein, a protein made in the liver involved in the transportation of thyroxine and retinol, misfolds. There are two forms of this subtype:
TTRm — The mutant / familial form in which there is a problem with the proteins themselves.
TTRwt — The wild-type (formally known as the senile form) etiology occurs when the protein itself is normal but gets misfolded
The reason it’s important to understand the different types of amyloid is because the workup and treatment are very different between the two.
In this picture, you can see how both the TTR and AL amyloid are created and deposit in the heart. The TTR tetramer protein is created in the liver. The tetramer dissociates, and then one of the proteins gather to create beta pleated sheets that the body cannot break down. In the AL version, the bone creates plasma cells that then create immunoglobulin. The light chains from the immunoglobulin come together to also form beta pleated sheets and deposit in the heart.
HFrEF GDMT Overview
Guideline-directed medical therapy (GDMT) is the foundation of treatment for HFrEF and includes renin-angiotensin-aldosteronesystem inhibitors, β-blockers, mineralocorticoid receptor antagonists, and sodium-glucose co-transporter 2 (SGLT2) inhibitors. Combined use of these four classes has been shown in major trials to significantly improve clinical outcomes, including morbidity, mortality, or hospitalizations.
GDMT Initiation/Optimization: GDMT should be continued, optimized, or initiated during hospitalization when possible
If pre-existing GDMT, continue and optimize to target doses unless contraindicated. Resume as soon as possible if discontinued.
Initiate GDMT inpatient once clinically stable
All four medications (in any order) should ideally be started within 4-6 weeks of initiation
ARNi is the recommended RAAS inhibitor for hospitalized patients with acute HF
Heart Failure Management Principles:
The table above lists the four pillars of GDMT, their function, indication, and common dosing. Note: Diuretics are NOT part of GDMT but do help symptoms!
Beta-blockers (three specifically - bisoprolol, metoprolol succinate, carvedilol)
Beta blockers should be started at diagnosis, including during hospitalization (once improved from initial decompensation due to risk of causing cardiogenic shock), and continued long-term even without symptom improvement.
RAAS inhibitors (ARNi, ACEi, ARBs)
For patients with Stage C HF and NYHA class II–III, ARNIs are first line. If patients have ever had angioedema with an ACEI, it is contraindicated. Notably, ARNIs also causes more potent BP reduction than ARBs or ACEi.
ARBs can be used in place of ACEis when cough or angioedema occurs, and a 36-hour washout is required when transitioning between an ACEi and an ARNi.
SGLT2i (empagliflozin, dapagliflozin)
Should be used in patients, regardless of if they have diabetes. Can increase risk of UTIs, so avoid in patients with history of frequent UTIs.
MRAs (spironolactone, eplerenone)
Use requires careful monitoring due to risks of renal dysfunction and hyperkalemia, and they are contraindicated in patients with an eGFR ≤30 mL/min/1.73 m² or serum potassium ≥5.0 mEq/L. Eplerenone, being more selective for the aldosterone receptor, is associated with fewer hormonal side effects like gynecomastia and vaginal bleeding compared to spironolactone.
*** GDMT initiation also depends on heart failure staging.
For ACC/AHA Stage B, the goal of medications is to prevent progression to symptomatic HF. At this stage, Beta blocker PLUS ACEi or ARB is indicated.
For ACC/AHA Stage C or D, the goal is to reduce morbidity, mortality, and hospitalization. All of the above GDMT medications are indicated.
This infographic above from CoreIM helps review the different pillars of GDMT.
Diuretics
Diuretics should be given to patients with signs of congestion, and maintenance therapy may be needed to prevent recurrence. The goal is to relieve fluid retention using the lowest effective dose. Loop diuretics are the primary choice for managing fluid overload. Thiazide diuretics may be used for patients with mild fluid retention and hypertension, while metolazone or chlorothiazide can be added for refractory edema. Diuretics improve symptoms but are not associated with mortality benefit and should be used alongside GDMT, not alone. If volume overload symptoms are very minimal, consider initiation of SGLT2i, MRA, and/or RAASi first, which may improve symptoms without needing an additional diuretic.
Diuretic strategy:
Start loop diuretics promptly (if they have a home dose, the rule of thumb is to double dose and deliver IV)
Consider ordering a spot urine sodium within the first two hours to assess the initial response to diuresis (urine Na <50 two hours after receiving a diuretic is associated with poor diuretic response and may suggest requiring dose escalation)
Monitor I/O strictly/UOP for response, vitals, and daily body weight
Regularly assess electrolytes and renal function (cardiorenal physiology may improve with diuretics as patient approaches euvolemia, but overdiuresis can result in hypoperfusion and a pre-renal AKI)
If a patient fails to respond to initial dose of diuretic within first 1-2 hours, immediately administer double the dose. If patients fail to respond to oral diuretic, this may be due to intestinal edema preventing absorption, and diuretics should be administered by IV route. For chronic suspicion of gut edema limiting diuretic absorption in the outpatient setting, torsemide can be considered with improved oral bioavailability.
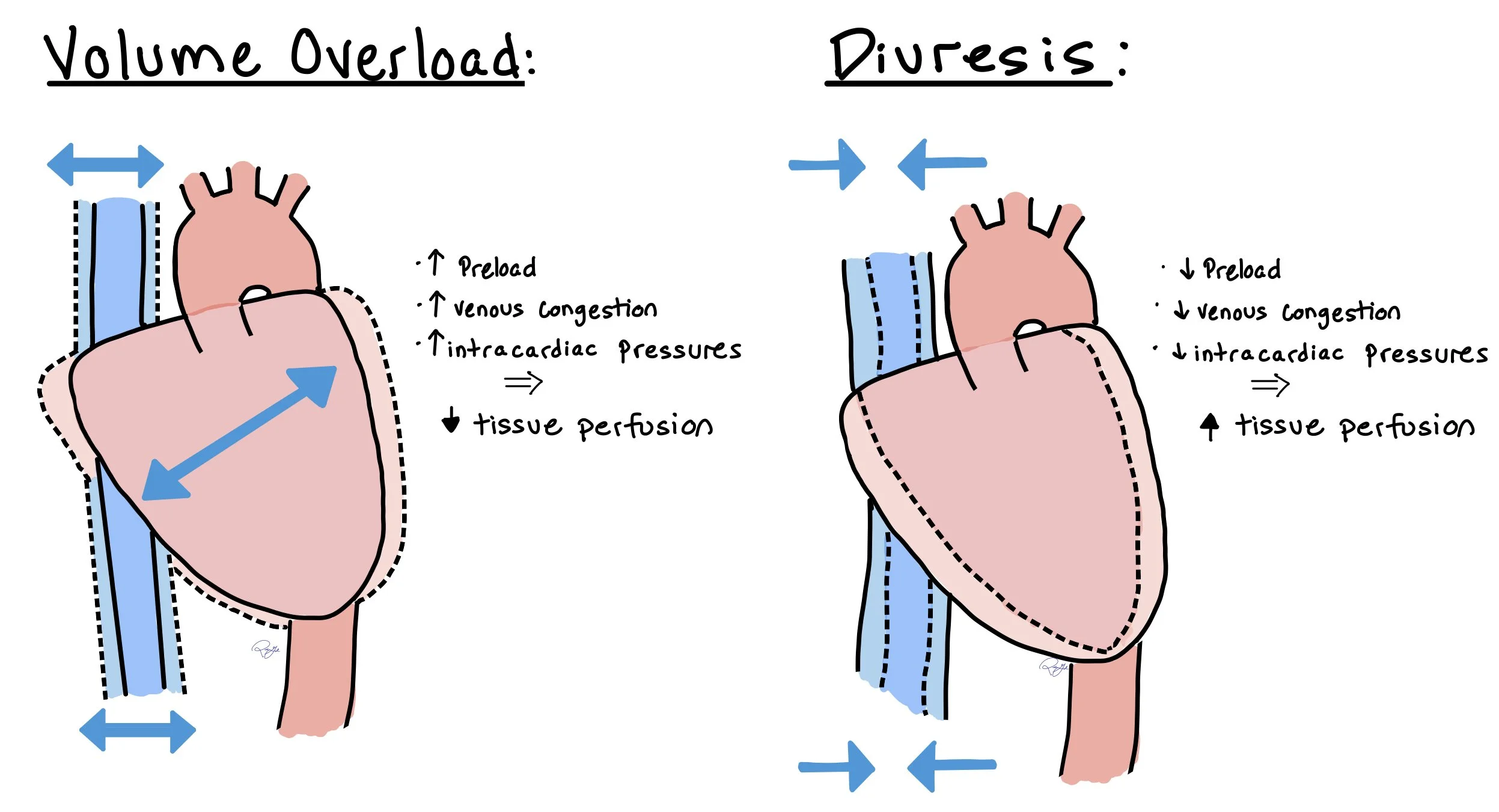
The figure above shows how increased volume in acute decompensated heart failure leads to higher preload, increased venous congestion, and increased intra-cardiac pressures. As a direct result, this leads to decreased tissue perfusion. When patients with acute decompensated heart failure receive diuresis, this will decrease preload, venous congestion, and intra-cardiac pressures, all leading to increased tissue perfusion.
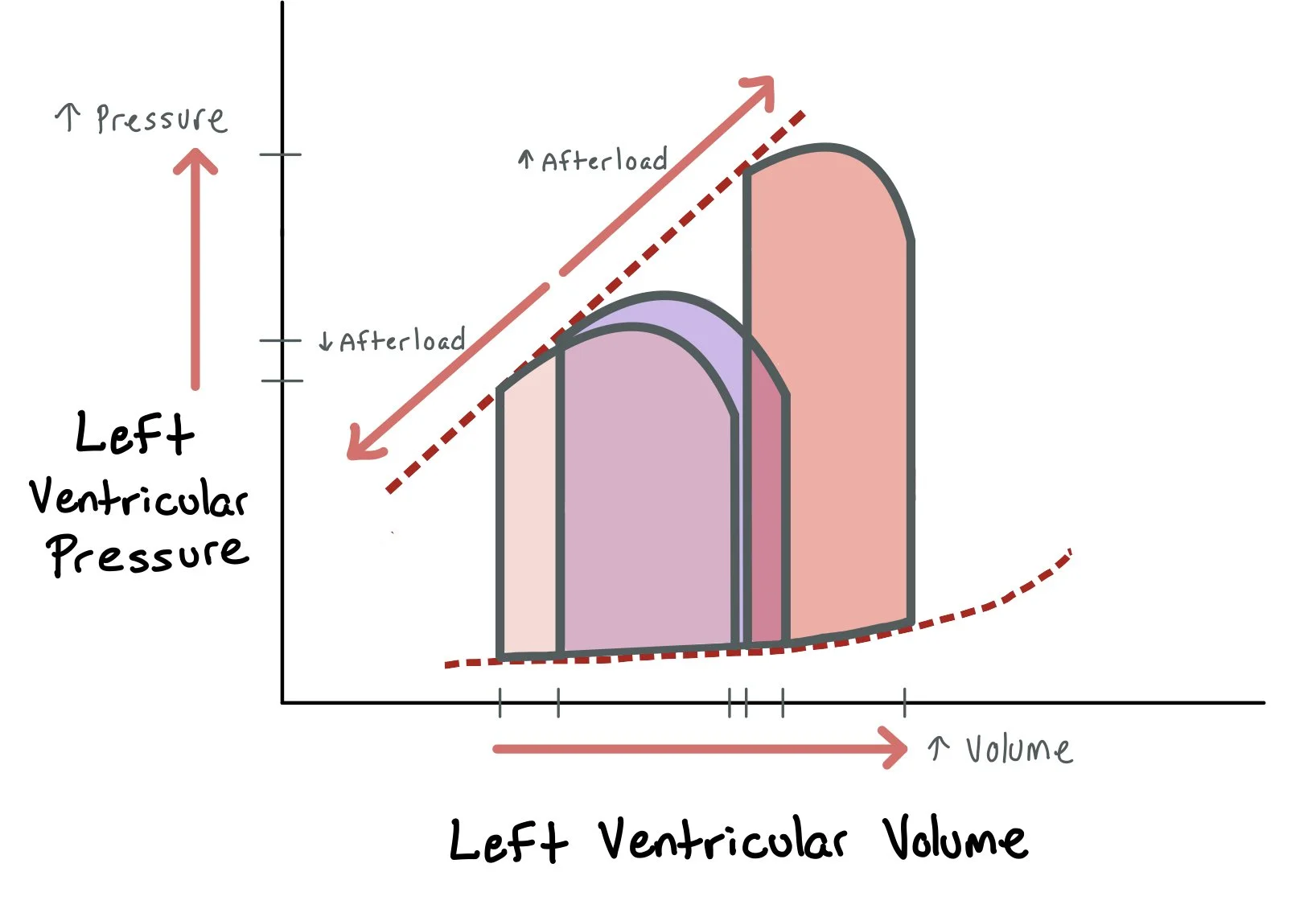
The pressure-volume loops above demonstrate how increased volume leads to increased left ventricular pressure and afterload. By using diuretics in patients with acute decompensated heart failure, we remove volume and, therefore, decrease LV pressure and afterload.
Assessing Cardiogenic Shock
Stages of CS (2019 SCAI SHOCK stages):
A (At risk): Not currently experiencing symptoms of CS (patients are warm and well-perfused) but at risk for developing it d/t having MI or acute-on chronic HF symptoms
Normal JVP, lactate, renal function, BP
CI > 2.5, PCWP< 15
B (Beginning CS): clinical evidence of HD instability (hypotensive/tachycardia) but still well-perfused
Elevated JVP, BNP, minimal renal function changes, hypotensive, tachycardia
Normal lactate
C (classic CS): hypoperfusion needing additional interventions, such as inotropes and mechanical cardiac support
Volume overload, overall looks unwell, AMS, cold and clammy, cool extremities, low UOP
Lactate > 2, SCr increase x 1.5 from baseline, increased LFTs, BNP
CI < 2.2 PCWP >15
D (deteriorating): progression from category C (initial support strategies utilized beyond fluid resuscitation are not helping) → needing higher doses of pressors/additional pressors
Lactate > 2 and progressively uptrending, deteriorating renal function, elevated LFTs, BNP
E (extremis): actual/impending circulatory collapse
Near pulselessness, cardiac collapse, multiple defibrillators
Lactate > 8, pH< 7.2
Classification of decompensated heart failure states:
4 presentations- treatment is dependent on presentation primarily assessing volume and perfusion status
Warm and wet: adequate perfusion with fluid overload
Warm and dry: adequate perfusion and no fluid overload
Cold and wet: inadequate perfusion with fluid overload*
Cold and dry: inadequate perfusion and no fluid overload*
*Concerning for cardiogenic shock
Remember: To treat the patient in cardiogenic shock with volume overload, you have to warm them up (inotropes) to dry them out (diuresis).
Back To The Case:
1. What is the differential diagnosis? What factors support one diagnosis as the most likely?
Any time a patient comes in with new dyspnea and edema, we have to think heart failure. However, note that other organ dysfunction, such as renal failure, liver failure, and lung pathologies can potentially present similarly! Remember that heart failure is a clinical diagnosis!! Taking a thorough history and doing a detailed physical exam and looking for JVD, crackles, lower extremity edema, and signs of inadequate perfusion (i.e. poor urine output, altered mentation, cool extremities, etc) are crucial! When ordering labs, you should get a BNP, troponin (if worried about ACS, PE, etc), CBC, BMP, iron studies, and TSH. Make sure to also order a UA and blood cultures if you are worried about infection, as this can cause heart failure!
If this is new heart failure, you can order a TTE to assess systolic and diastolic function, as well as any underlying valvular issues. Regional wall motion abnormalities can be suggestive of ischemic disease Chest x-ray: often demonstrates cardiomegaly and pulmonary vascular congestion.
Finally, get a 12-lead ECG and make sure to look at it yourself! While it can often be normal, it may provide insight into underlying etiology or comorbid cardiac pathologies including ischemic events
2. How is heart failure classified, and why does it matter?
When we talk about heart failure, it’s important to classify it as ischemic (i.e due to coronary artery disease) or non-ischemic. From there, we use staging to assess the degree of heart failure (i.e whether the patient is at risk [Stage A], is starting to have signs of heart failure on labs / imaging [Stage B], has symptoms [Stage C], or is end stage [Stage D]). It’s important to also report the patient’s NYHA functional status as it allows us to know how decompensated the patient is. Assessing stage and functional status is crucial as we give our GDMT based on these factors! Note: Staging can only get worse while functional status can get better or worse depending on the patient’s level of decompensation (ex. once a patient is diagnosed as Stage C, they can never go back to Stage B).
3. How do we approach diuresis and volume management in this patient?
With diuresis, we usually give the patient 1.5 - 2x their home dose of IV diuretics. Usually, oral diuretics will not be effective as these patients have gut edema, decreasing the diuretic absorption. We aim for 100-200mL output within the first 1-2 hours of diuretic dosing. If the patient does not urinate, double the dose! See When the Beat Drops: Diuretics chapter coming soon!!
4. How do we initiate and titrate GDMT in a patient with newly diagnosed HFrEF? What additional medications and advanced therapies can be used for symptom management in heart failure?
Initiating GDMT for HFrEF may differ between patients as some may be more sensitive to each medication. GDMT includes renin-angiotensin-aldosteronesystem inhibitors, β-blockers, mineralocorticoid receptor antagonists, and sodium-glucose co-transporter 2 (SGLT2) inhibitors. Combined use of these four classes has been shown in major trials to significantly improve clinical outcomes, including morbidity, mortality, or hospitalizations.
GDMT Initiation/Optimization: GDMT should be continued, optimized, or initiated during hospitalization when possible
If pre-existing GDMT, continue and optimize to target doses unless contraindicated. Resume as soon as possible if discontinued.
Initiate GDMT inpatient once clinically stable
All four medications (in any order) should ideally be started within 4-6 weeks of initiation
ARNi is the recommended RAAS inhibitor for hospitalized patients with acute HF
Further Learning:
Pearls and Pitfalls of GDMT in the hospital:
***OPTIMIZE GDMT AS MUCH AS POSSIBLE WHILE INPATIENT BEFORE THE PATIENT LEAVES!! This has been shown to be the MOST clinically beneficial intervention for heart failure!!!
Consider holding beta blocker therapy on admission if…
There is a question of medication adherence as initiating therapy may precipitate decreased cardiac output and hypoperfusion (shock)
Shock (cardiogenic, septic, or otherwise), or other states in which the body may require increased cardiac output for adequate perfusion
Symptomatic bradycardia
If a patient comes in with…
Elevated K+ (>5-5.5) or a significant acute kidney injury: Hold ACEi/ARB/ARNi, MRA
Hypotension: ACEi/ARB/ARNi have the strongest blood pressure effect, so consider holding these medications
Urinary tract infection: Hold SGLT-2, but if it is an uncomplicated UTI or there is a clear alternate precipitating factor, consider resuming on discharge with close monitoring unless complications become recurrent
Angioedema: Hold ACEi, ARB, ARNi as these medications (particularly ACEi) can trigger angioedema episodes
Further Learning
Strong HF — An intensive treatment strategy of rapid up-titration of guideline-directed medication and close follow-up reduced the risk of 180-day all-cause death or HF readmission compared to usual care.
Core IM 5 Pearls on GDMT Part 1 and Part 2
Core IM 5 Pearls on Inpatient Heart Failure
DAPA-HF (2019) — In patients NYHA class II-IV with EF<40% the use of dapagliflozin decreased the risk of worsening heart failure or death from cardiovascular cause regardless of co-existing diabetes diagnosis
PARADIGM-HF (2014)— This trial compares sacubitril-valsartan (ARNi) with enalapril (ACEi) in patients with symptomatic HFrEF. In patients who tolerate target dosing of ACEi/ARB, use of sacubitril-valsartan reduced cardiovascular death or heart failure hospitalization by 20% compared to enalapril
RALES (1999) — The addition of spironolactone to standard therapy reduces the risk of morbidity and mortality in patients with heart failure. Notably, standard therapy at the time of this trial was ACEi, loop diuretic, and in some cases digoxin
Remember: Patients with HFrEF <40 should preferentially be put on an ARNI!!
How’d we do?
The following individuals contributed to this topic: Atreya Mishra, MD*; Savina Sahgal, MD*; Pal Shah, MD*; Michael Hill, MD
Chapter Resources
https://www.ahajournals.org/doi/10.1161/CIR.0000000000001063
2022 AHA/ACC/HFSA heart failure guidelines
Overview of cardiogenic shock
SCAI classification of cardiogenic shock
State of the art review of hemodynamics
Brief overview of PA catheter placement
https://www.ahajournals.org/doi/10.1161/CIRCHEARTFAILURE.120.007099#F1
Use of PA catheter data for appropriate classification of cardiogenic shock
Cardionerds Podcast on RHC evaluation in cardiogenic shock