Case Presentation:
A 58-year-old male presents via EMS with SOB, palpitations, and dizziness and is found to be in atrial fibrillation with rapid ventricular response (afib with RVR). The patient has a PMH of HFimpEF (EF: 50%) on GDMT (ARNI, BB, SGLT-2-I, and MRA), CAD- MI x4yrs ago s/p PCI, HTN, and T2DM. The patient was initially treated in the ED before being transferred to Cardiology for management of his rapid heart rate.
Vitals in the ED: Temp 99.1F BP 134/98 mmHg HR 162 bpm RR 26 O2: 94% on RA
Notable Labs: HS troponin peak 130 ng/L (normal <15-20), BNP 470 pg/mL (normal <100), Cr 1.7 (baseline 1.2 mg/dL), Lactate 2.4 mmol/L. D-dimer 200 ng/mL (normal <500).
EKG: Afib with rates in the 150s. Normal axis / intervals. No ST-elevations / depressions or T wave inversions
Bedside TTE: LV with overall good squeeze. IVC > 2.5 cm with no inspiratory variation
ED course: diltiazem 20mg (0.25mg/kg) IV Push and diltiazem continuous infusion was started at 10mg/H
*** 12 hours after the diltiazem infusion was started, the nurse calls an RRT for hypotension.
Vitals at the RRT: Temp 98.8F BP 74/52 mmHg HR 112 bpm RR 35 O2: 92% on RA
Labs: SCr: 2.3 mg/dL, lactate 3.4 mmol/L, troponin 240 ng/L, BNP 910 pg/mL, glucose: 127
Bedside TTE: LVEF significantly reduced and estimated at < 15%
Physical exam:
HENT: dizziness, + JVD
Pulmonary: diffuse bilateral crackles
Extremities: 2+ pitting edema up to bilateral knees
Skin: cool extremities with diminished pulses
The patient is immediately brought to the cath lab and Swan Ganz catheter is placed:
Right Atrial Pressure: 16 mmHg, SVR (systemic vascular resistance): 1400 dynes-sec/cm5, PCWP (pulmonary capillary wedge pressure): 34 mmHg, CI: 1.6 L/min/m2 by Fick, PA pressure: 42/20 (mean 33), Pulmonary Vascular Resistance: 4 mmHg min/L
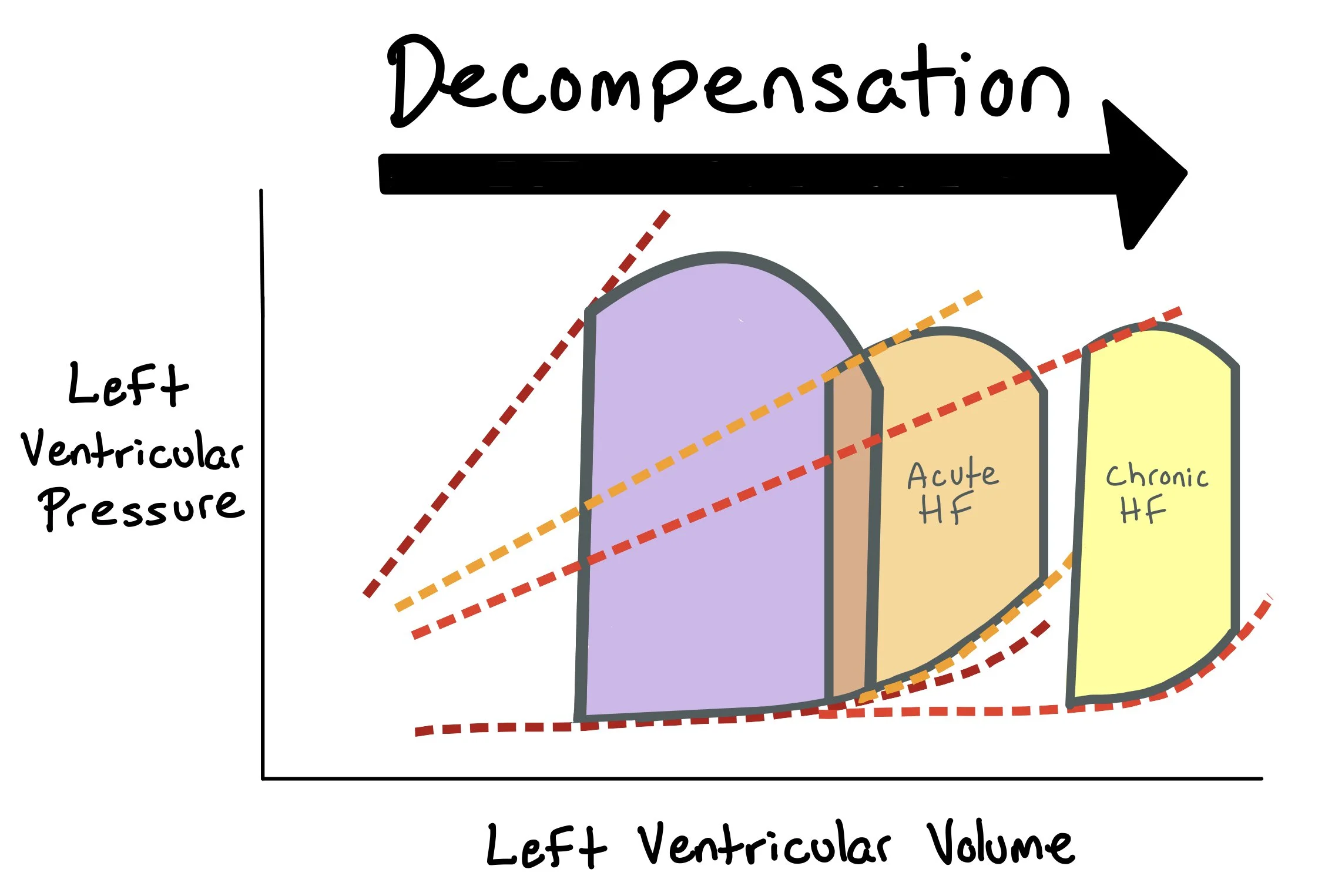
The picture above shows pressure-volume (PV) loops for patients with normal heart squeeze (purple) vs. those with acute heart failure (orange) and those with chronic heart failure (yellow).
Questions:
How do you determine what type of shock this patient has? What are you specifically looking for on your labs, imaging, and physical exam to support your assessment?
What is your initial approach to treating this patient? How do you decide which inotropic and vasopressor agents to treat this patient with?
What additional therapies should you consider if the patient requires additional support?
Different Types of Shock:
Different types of shock
Shock is the inability of the circulation to sustain cellular respiration needed to maintain normal organ function. When patients are in shock, they have decreased perfusion to vital organs, resulting in multiorgan dysfunction and eventual death if not corrected. As a result, they will have arterial hypotension, tissue hypoperfusion, and lactic acidosis.
Etiologies of shock can be divided into a few main categories, namely:
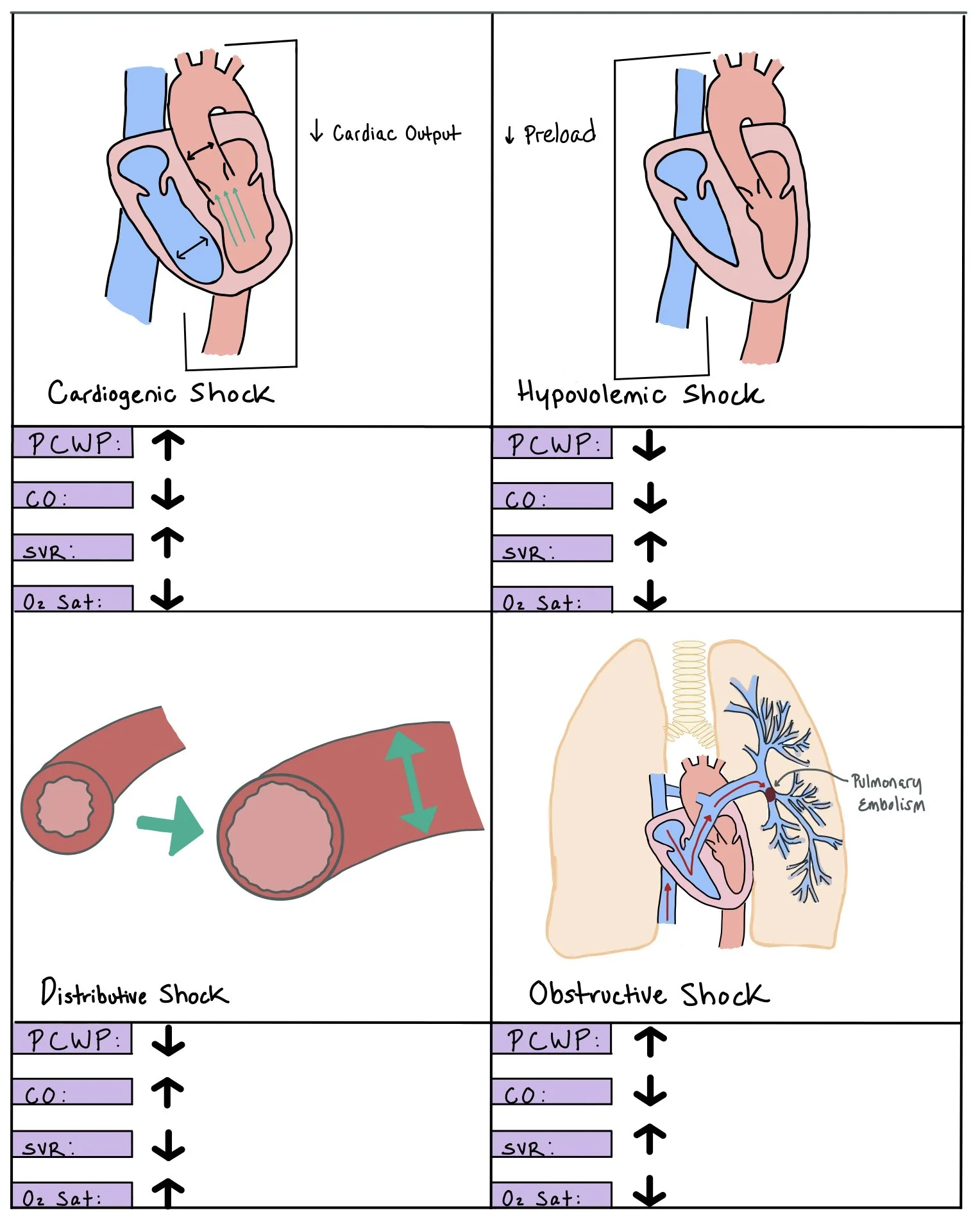
Hypovolemic
Occurs when there is decreased intravascular volume and increased systemic venous compensation to maintain perfusion during the early stages of shock. Hypovolemic shock can be divided into two categories, hemorrhagic and non-hemorrhagic shock (i.e. GI wasting, third spacing, insensible loses).
Cardiogenic
Cardiogenic shock is defined as insufficient cardiac output to meet the basic metabolic requirements causing tissue hypoxia due from circulatory failure. This occurs when there is a reduction in myocardial contractility that leads to increased venous congestion, decreased cardiac output (CO), hypotension, systemic vasoconstriction, and cardiac ischemia.
Obstructive
Due to any etiology that causes decreased left ventricular output. Examples of obstruction can be from mechanical obstruction (i.e. pulmonary embolisms or left ventricular outflow track obstruction), restrictive obstruction (i.e. tension pneumothorax, cardiac tamponade, restrictive cardiomyopathy)
Vasodilatory
Also known as distributive or septic shock, this type of shock is due to systemic dilation of the vascular system, resulting in a low systemic vascular resistance (SVR). Vasopressors, which help to increase peripheral vascular resistance, should be added in these cases.
This picture depicts the different types of shock, including cardiogenic, hypovolemic, distributive, and obstructive shock. Each panel shows how the specific shock affects the pulmonary capillary wedge pressure (PCWP), cardiac output (CO), systemic vascular resistance (SVR), and O2 saturation.
Pro-Tip: The fastest way to determine if the patient will be fluid responsive is to put the patient into Trendelenburg (i.e feet up higher than the head). If the blood pressure increases in this position, they will most likely respond well to fluid. If the blood pressure does not improve, this may suggest that their problem will not be resolved by giving fluid.
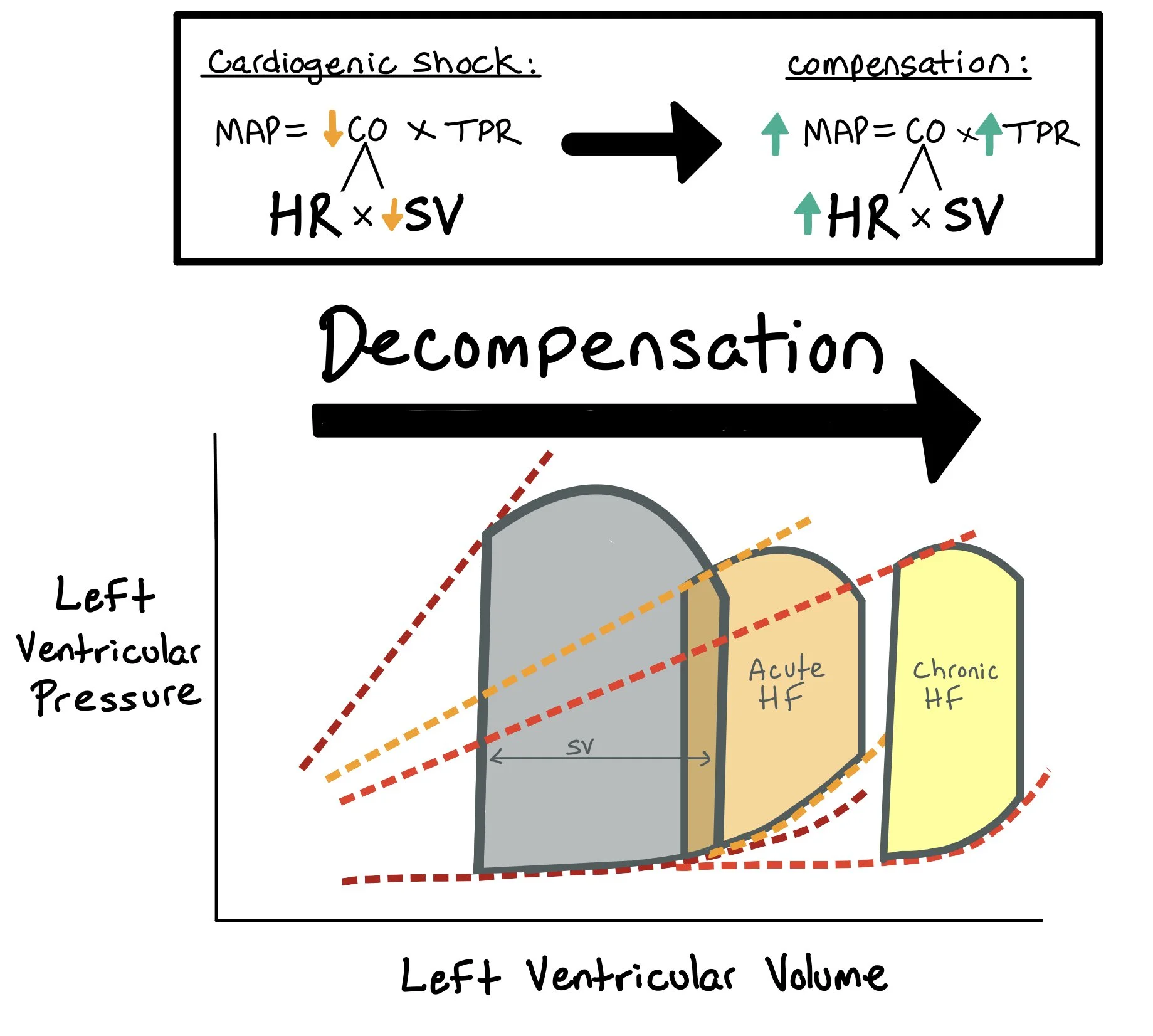
We must recognize when patients are in both cardiogenic and vasodilatory shock so that we can appropriately treat them. When patients are in cardiogenic shock, the mean arterial pressure (MAP) decreases because stroke volume (SV) decreases. As a compensatory response, the body will increase the systemic vascular resistance (SVR) to help protect the MAP as low MAPs lead to poor perfusion and organ damage.
** Note: Just because someone has a normal MAP (>65) does not mean that they aren’t in cardiogenic shock!!! This would be categorized as normotensive cardiogenic shock. One should look at pulse pressure and proportional pulse pressure -- both of which are surrogates for stroke volume and help identify this “normotensive” cardiogenic shock.
When patients with cardiogenic shock develop systemic vasodilation, their SVR plummets. Suddenly, the patient has lost their ability to compensate, which leads to very poor outcomes. We can determine whether a patient is in this mixed shock state most easily by assessing their cardiac pressures, mixed venous (SV02), and SVR through a Swan Ganz Catheter. We can also use the central venous pressure and SV02, doppler echocardiography low cardiac output and low systemic vascular resistance, and physical exam with assessing pulse pressure and extremity warmth and pulses.
It is important to note that patients can develop more than one type of shock at a time. In the CCU, we may see a combination of cardiogenic and vasodilatory. Patients may initially have only one type of shock but, due to decompensation, infection, or disease progression, they develop an additional shock state. Here are a few examples:
Cardiogenic – Vasodilatory Shock
The patient starts with cardiogenic shock but develops a vasodilatory shock due to an overlying infection or inflammatory state (think acute MI, myocarditis, etc). Diffuse inflammation in the body causes reactive oxygen species to accumulate which ultimately leads to nitric oxide formation and systemic vasodilation.
Vasodilatory – Cardiogenic Shock
The patient will be in vasodilatory (or septic) shock due to an infection but develops concurrent cardiogenic shock due to the heart’s inability to keep up with the vasodilatory shock state. When the patient is in vasodilatory shock, this increases the demand on the heart – unfortunately, if the patient has a weaker heart to begin with, the heart may not be able to help keep up and compensate for the shock state.
Primary Mixed Shock
In this shock state, patients concurrently develop both cardiogenic and vasodilatory shock at the same time. A prime example of this is post- cardiac arrest when the heart is in shock and the systemic inflammation from the arrest causes systemic inflammation and dilation (aka vasodilatation).
Note: When a patient is in cardiogenic shock, they have decreased CO due to the decreased SV. In these cases, the patient is dependent on the HR to help compensate for the low SV and to maintain the CO. Giving patients with decompensated heart failure medications to decrease the HR (specifically in afib with RVR) can cause severe decompensation.
Helpful initial labs and imaging:
Labs: CBC, BMP, LFTs, troponin, BNP, lactic acid, TSH, cortisol, blood cultures x2, urinalysis and culture. If the patient has central access, consider getting a mixed venous.
Imaging: EKG, CXR, CT angiography (if concerned for PE), right heart catheterization (RHC) to help assess type of shock if needed, left heart catheterization (LHC) if concerned for acute plaque rupture and MI causing shock.
Pressure-Volume Loops:
In order to know how to treat patients who come in with shock, it’s important to be able to understand the Pressure- Volume (PV) Loops and be able to conceptualize how different interventions change the curves:
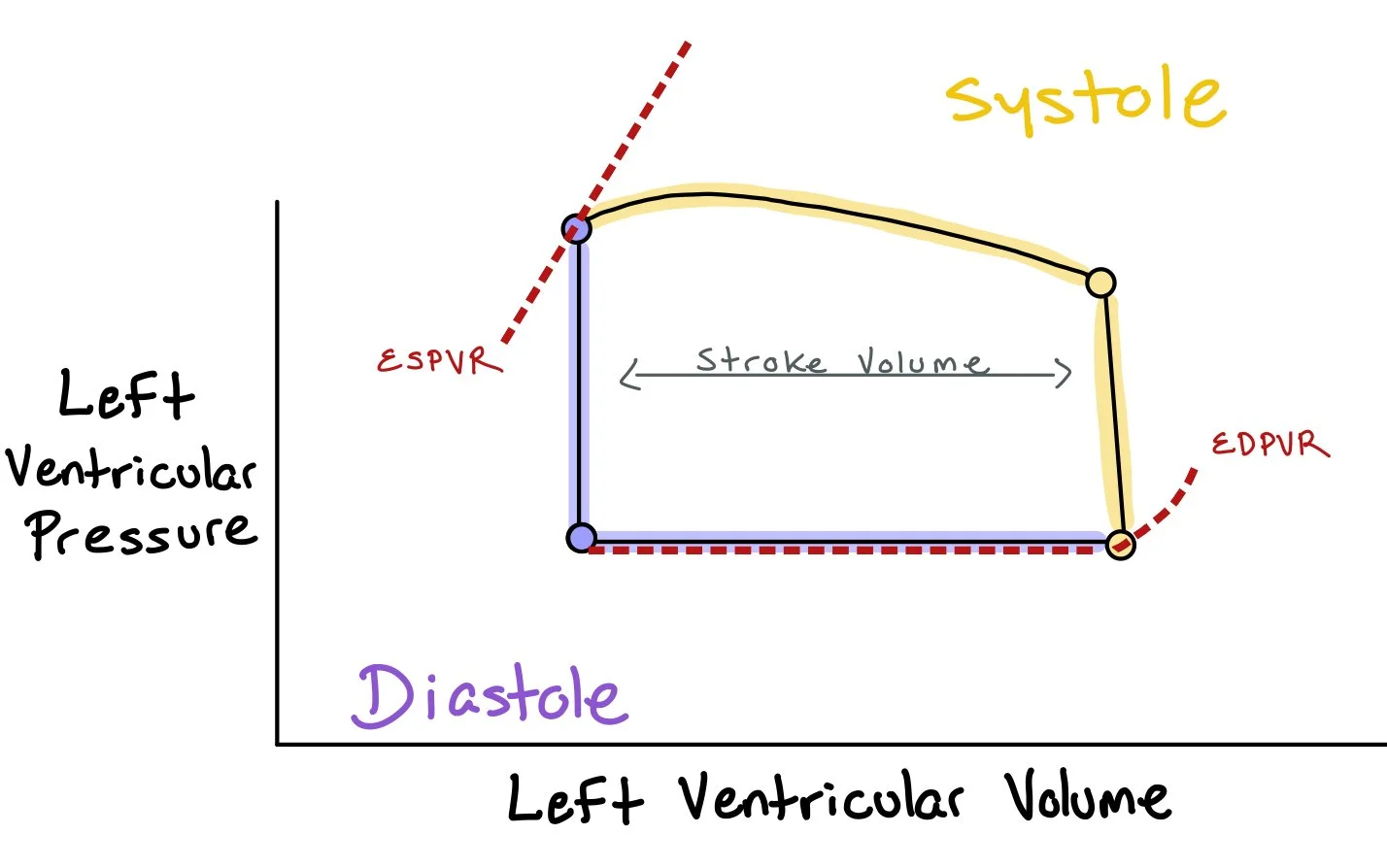
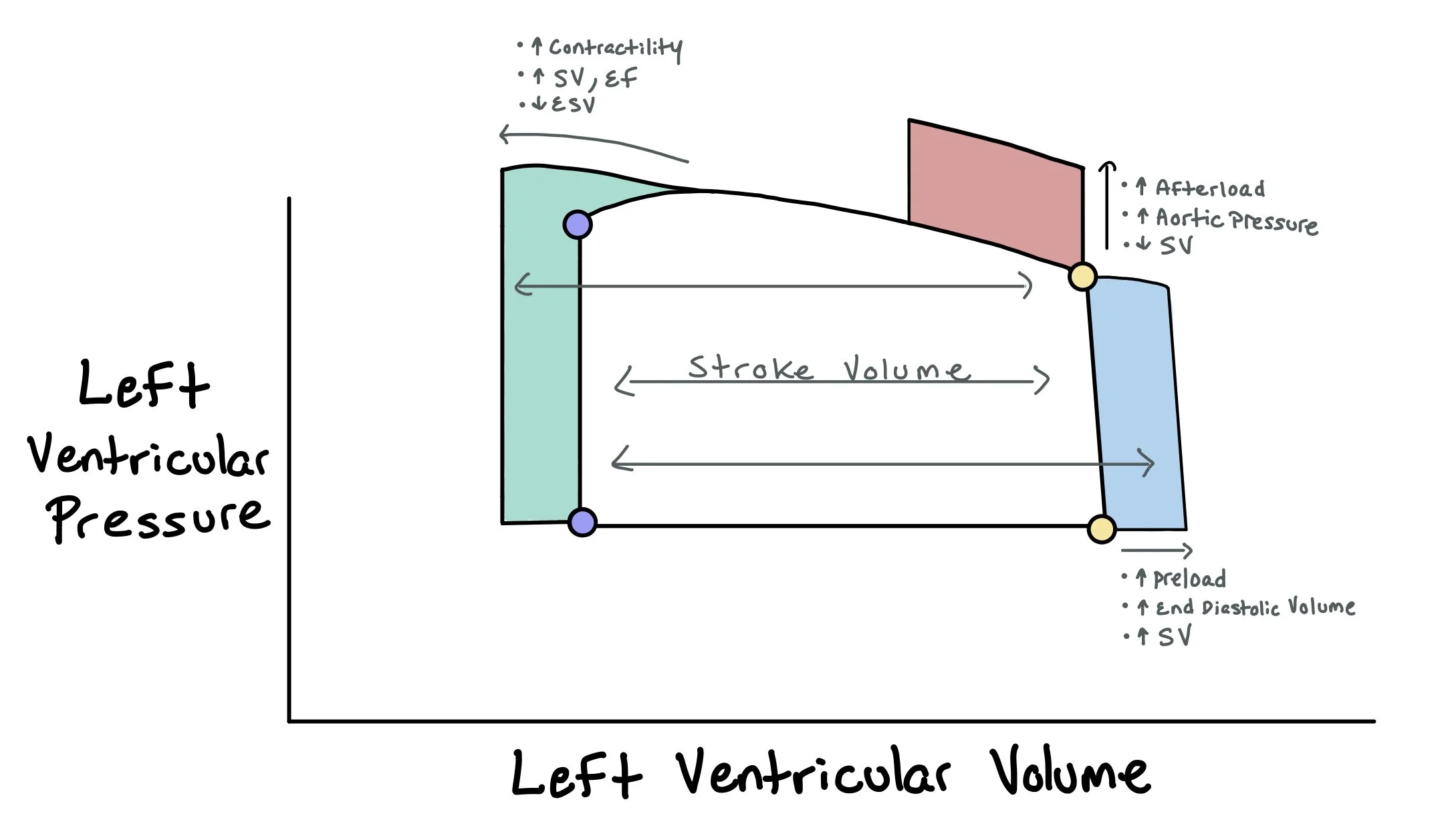
This picture depicts how the pressures in the left ventricle change as the heart fills in diastole and empties during systole. Notably, the difference between the volume at end diastole and end systole is the stroke volume (i.e. the amount of blood actually pushed out of the heart). The picture includes the end diastolic pressure-volume relationship (EDPVR), which represents the pressure at the end of diastole when the heart is full. Conversely, the picture also shows the end systolic pressure-volume relationship (ESPVR) that represents the pressure in the heart at the end of systole when the pressures in the heart are at the highest.
Now that we understand the basic PV loop, we can start to build upon those concepts to understand how these curves change in different situations, such as increasing or decreasing preload, afterload, or contractility.
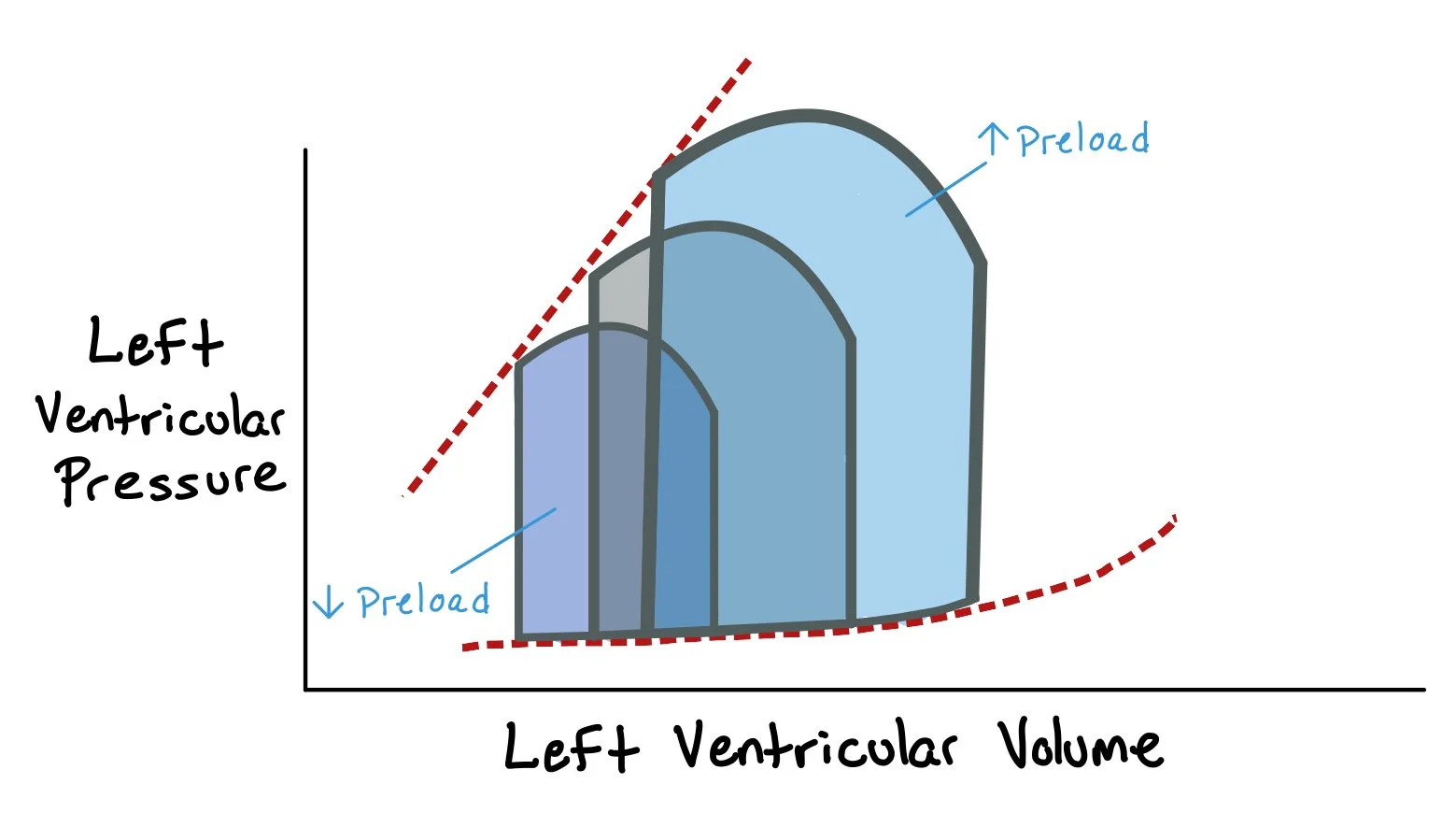
This graph shows how increasing preload, by adding more volume, leads to increased end diastolic volume and left ventricular systolic and diastolic pressures. As a direct result, the stroke volume (i.e. the difference between the end diastolic volume and end systolic volume) increases.
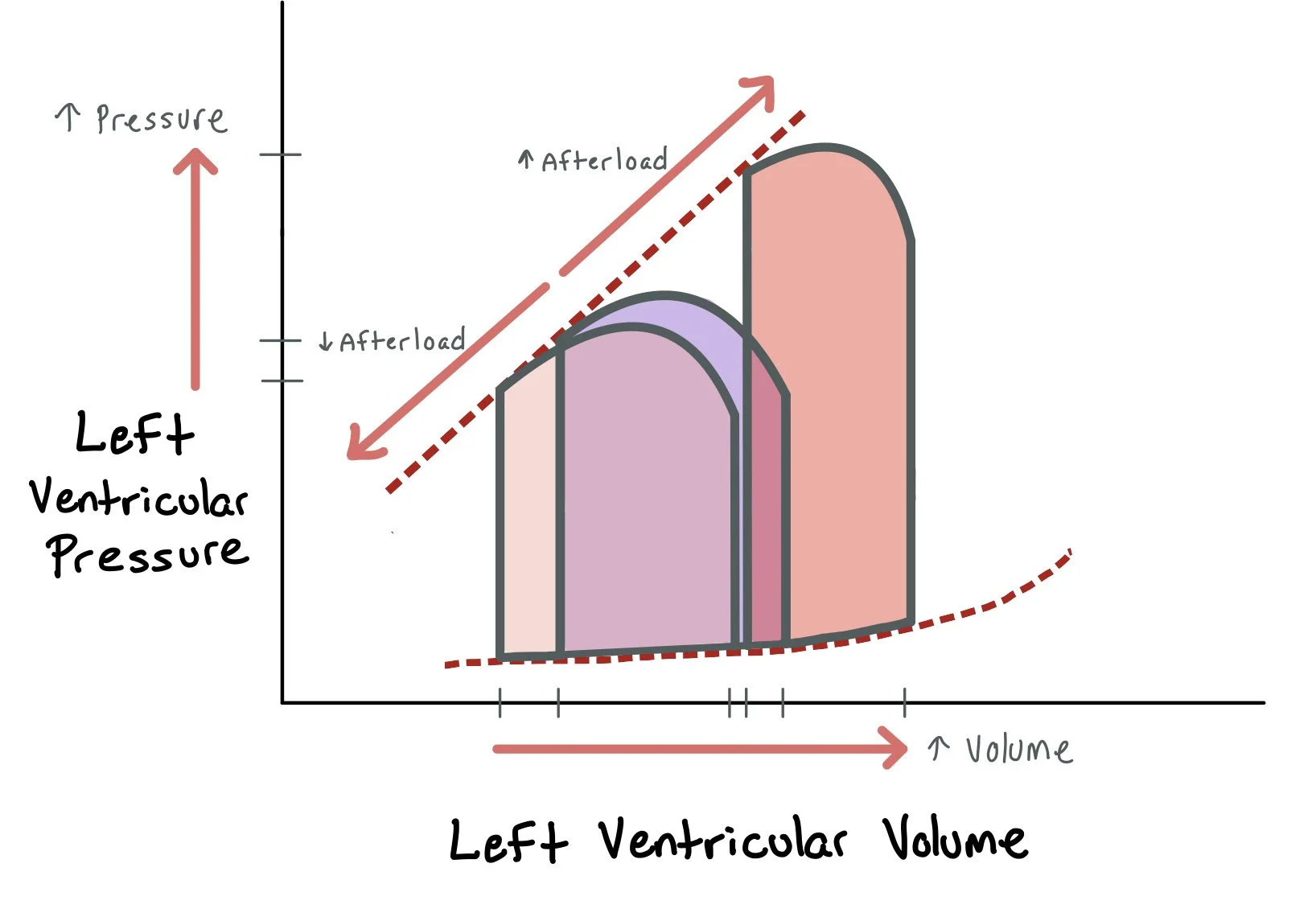
Afterload, or the resistance the ventricle must overcome to eject blood, also has a critical impact on PV loops. An acute increase in afterload (e.g., due to hypertension or aortic stenosis) increases the pressure required to open the aortic valve and shifts the upper right corner of the loop upward. This leads to increased ESV and reduced stroke volume—making the loop taller and narrower. Conversely, afterload-reducing agents (like ACE inhibitors, hydralazine, or nitrates) lower systemic vascular resistance, allowing the heart to eject more easily. This shifts the upper left corner of the loop downward and leftward, decreasing ESV and increasing stroke volume, especially in systolic heart failure.
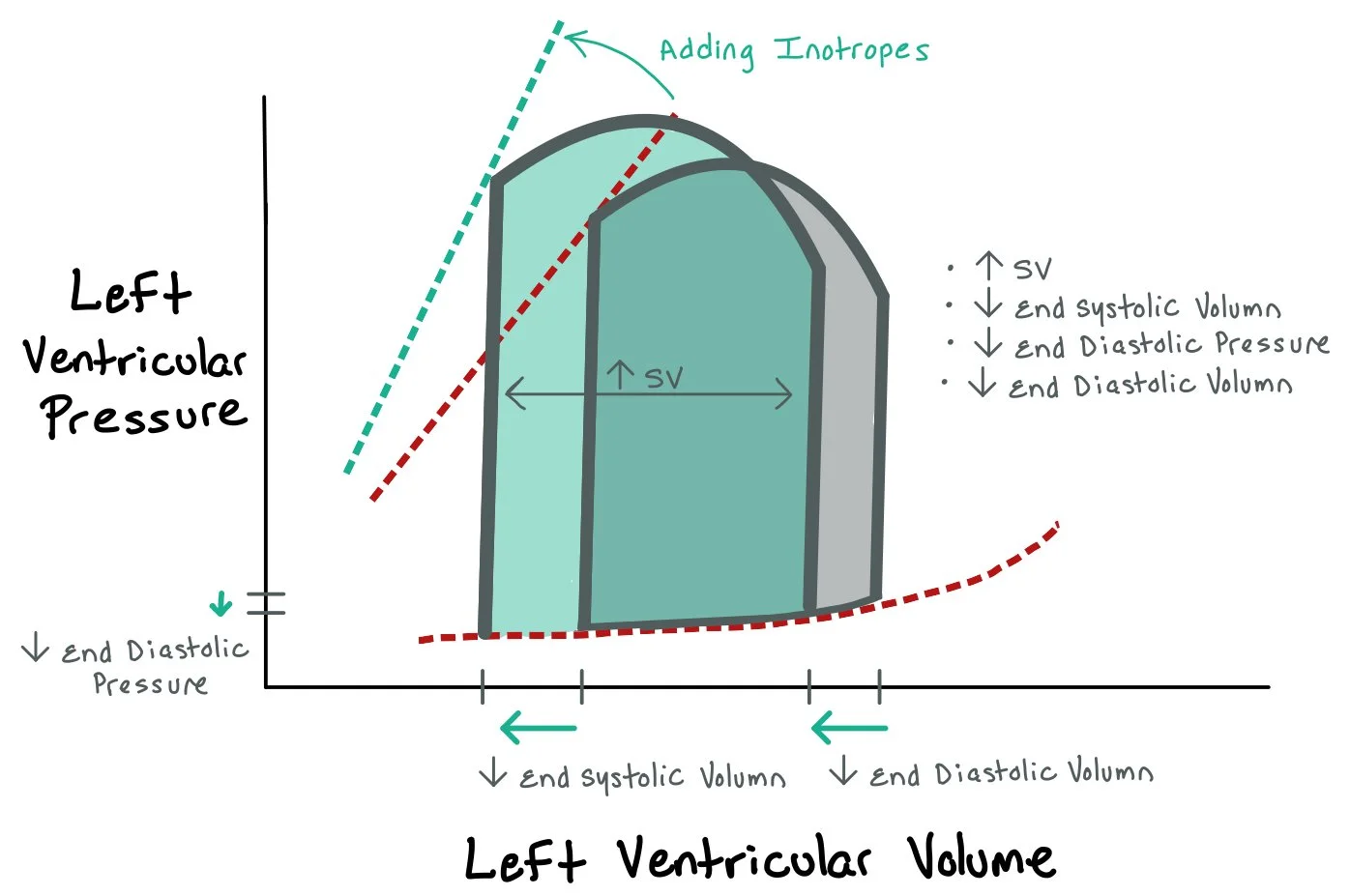
This graph depicts how adding inotropes that increase the contraction of the heart causes increased stroke volume since more blood is pumped out of the heart. This results in greater pressure generation during systole, more complete ejection of blood, and a decrease in end-systolic volume (ESV), thereby increasing stroke volume (SV). On the PV loop, these changes appear as a leftward and upward shift, with the loop becoming narrower and taller, indicating improved cardiac output. This response is especially beneficial in acute decompensated systolic heart failure, where baseline contractility is low and the loop is wide and flat. However, because inotropes increase myocardial oxygen demand and may provoke arrhythmias, they are typically reserved for short-term use in critically ill patients or as a bridge to more definitive interventions.
Putthing what we learned above together, we can now better understand the PV-loops for heart failure.
In acute heart failure, there is an abrupt decrease in cardiac contractility. This is represented by a downward shift in the end-systolic pressure-volume relationship (ESPVR), indicating the ventricle can no longer generate the same pressure at a given volume. As a result, end-systolic volume (ESV) increases due to incomplete ejection, and stroke volume (SV) decreases, producing a narrower loop. End-diastolic volume (EDV) may also increase due to the accumulation of blood that wasn’t ejected, and the loop shifts rightward. Clinically, this corresponds to signs of low cardiac output and congestion, such as pulmonary edema and hypotension.
In chronic systolic heart failure (heart failure with reduced ejection fraction, or HFrEF), the decline in contractility persists over time, leading to ventricular dilation and remodeling. The ESPVR remains depressed, and both ESV and EDV are elevated. The stroke volume is reduced, resulting in a wider and shorter loop. Chronic compensatory mechanisms, such as activation of the renin-angiotensin-aldosterone system (RAAS), increase preload and afterload, which initially help maintain output but ultimately worsen ventricular performance. The PV loop shifts to the right and becomes more flattened, indicating poor systolic function and volume overload.
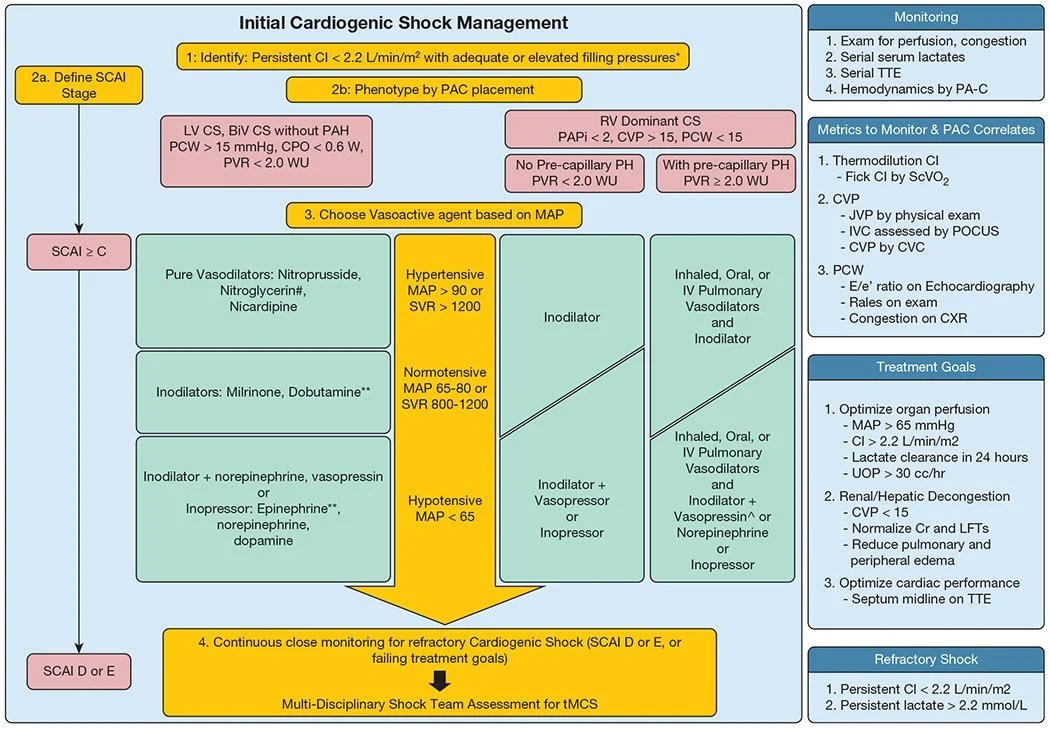
Assessing Cardiogenic Shock
Classification of decompensated heart failure states:
4 presentations- treatment is dependent on presentation primarily assessing volume and perfusion status
Warm and wet: adequate perfusion with fluid overload
Warm and dry: adequate perfusion and no fluid overload
Cold and wet: inadequate perfusion with fluid overload*
Cold and dry: inadequate perfusion and no fluid overload*
*Concerning for cardiogenic shock
Remember: To treat the patient in cardiogenic shock with volume overload, you have to warm them up (inotropes) to dry them out (diuresis).
Stages of CS (2019 SCAI SHOCK stages):
A (At risk): Not currently experiencing symptoms of CS (patients are warm and well-perfused) but at risk for developing it d/t having MI or acute-on chronic HF symptoms
Normal JVP, lactate, renal function, BP
CI > 2.5, PCWP< 15
B (Beginning CS): clinical evidence of HD instability (hypotensive/tachycardia) but still well-perfused
Elevated JVP, BNP, minimal renal function changes, hypotensive, tachycardia
Normal lactate
C (classic CS): hypoperfusion needing additional interventions, such as inotropes and mechanical cardiac support
Volume overload, overall looks unwell, AMS, cold and clammy, cool extremities, low UOP
Lactate > 2, SCr increase x 1.5 from baseline, increased LFTs, BNP
CI < 2.2 PCWP >15
D (deteriorating): progression from category C (initial support strategies utilized beyond fluid resuscitation are not helping) → needing higher doses of pressors/additional pressors
Lactate > 2 and progressively uptrending, deteriorating renal function, elevated LFTs, BNP
E (extremis): actual/impending circulatory collapse
Near pulselessness, cardiac collapse, multiple defibrillators
Lactate > 8, pH< 7.2
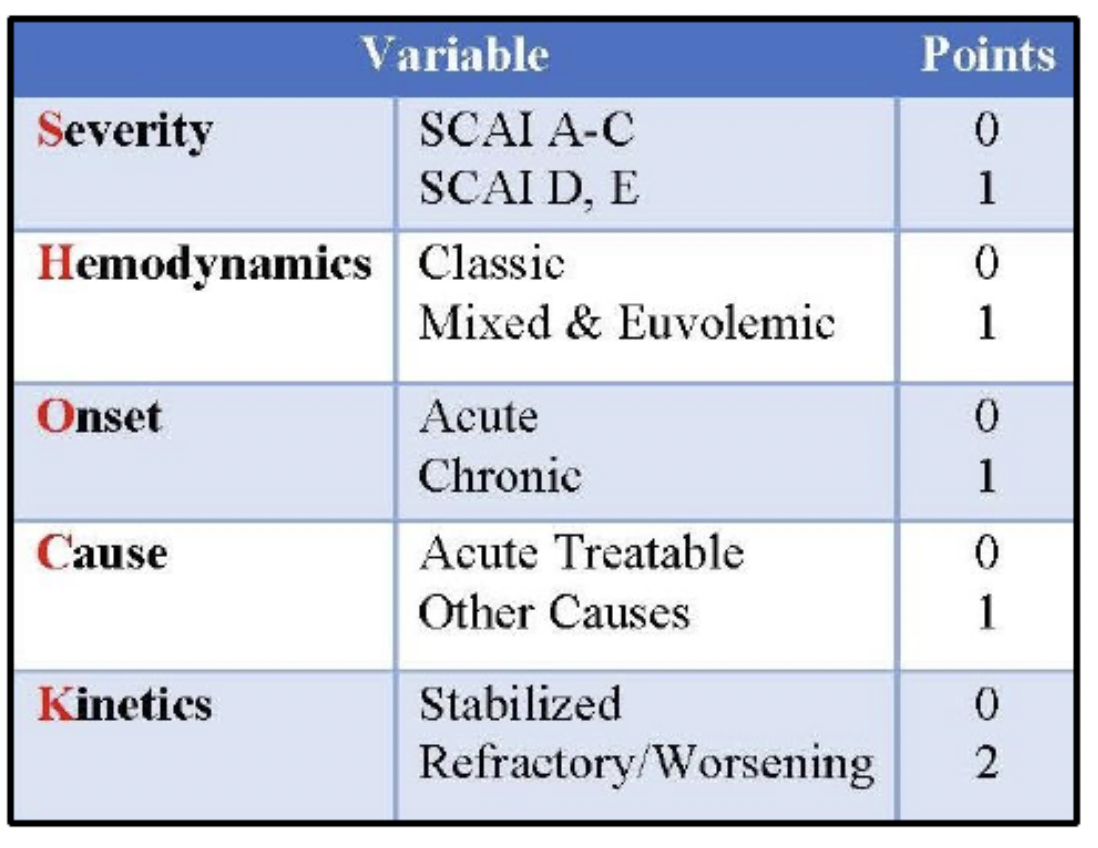
Given the complexity of cardiogenic shock with its wide spectrum of presentation, the Houston SHOCK Score was created. This score takes into account the different cardiogenic shock phenotypes, onset of pathology, kinetics, and hemodynamic profiles. By doing a more comprehensive risk evaluation, the SHOCK Score helps to grade the shock severity with the hopes of more quickly recognizing and treating the patient’s shock. This can be crucial when patients are not at a shock center and would benefit from early transfer to a tertiary center. The SHOCK Score grades Severity, Hemodynamics, Onset, Cause, and Kinetics of the shock. The higher the score, the higher the acuity of the patient and the worse the inpatient and long-term outcomes.
These two figures from Jumean et al. in the Journal of Shock and Hemodynamics overview the SHOCK Score, which is an important for grading and recognizing the acuity of patients with cardiogenic shock.
How to Differentiate Cardiogenic vs Septic Shock:
When patients are in shock, they have increased metabolic states. As such, they require more energy, aka ATP. Since aerobic metabolism gives the body more ATP, the body will try to deliver more oxygen. Figure 4 depicts the difference between cardiogenic vs septic shock and how the body tries to compensate in each case. The body has a few ways to help accomplish this:
1. Increasing cardiac output (CO) by increasing heart rate (HR) and stroke volume (SV).
Cardiogenic shock: the heart cannot generate enough force to increase stroke volume, so it must rely on the heart rate to increase CO. Clinically this is seen by narrow pulse pressures and is why patients in decompensated heart failure do very poorly with newly added beta blockers.
Septic shock: both HR and SV increase. Plus, the decreased systemic vascular resistance (SVR) allows for an increased CO.
2. Increasing oxygen absorption from the passing red blood cells
Cardiogenic shock: the body will try to take every last O2 from the red blood cells (RBCs).
Septic shock: given the increased CO, the tissues don’t have enough time to take O2 from the passing RBCs since they’re moving so quickly
3. Anaerobic metabolism
When all else fails, the body will go into anaerobic metabolism. This can be seen clinically with a rising lactic acid (LA) as this is the byproduct of anaerobic metabolism.
Choosing Diuretics
The role of diuretics in cardiogenic shock is alleviating vascular congestion, as this has been associated with worse outcomes including increased cardiogenic shock severity and mortality. Choice of diuretics should include loop diuretics, escalating doses until adequate urine output is achieved. If inadequate response to monotherapy loop diuretics at high doses (loop diuretic resistance), can add thiazide/thiazide like diuretics to get a sequential nephron blockade and/or administer the loop diuretic as a continuous infusion. Loop diuretics work on the Loop of Henle and thiazide/thiazide-like diuretics work on the distal tubule and have synergistic effect to allow for more effective diuresis, however if both diuretics are being utilized due to more effective diuresis, there is an increased likelihood of electrolyte abnormalities and should be monitored closely.
A general rule of thumb to properly dose diuretics include doubling the patients home diuretic dose and administering it intravenously. If an adequate urine output is achieved, can schedule 2-3x daily. Monitoring of diuretics includes urine output (UOP) and electrolytes and can titrate up the dose or consider a continuous infusion to reach a goal of more than 150mL/hour. Additionally, a spot urine sodium of > 70 mmol/L has also been shown to correlate with appropriate diuretic response.
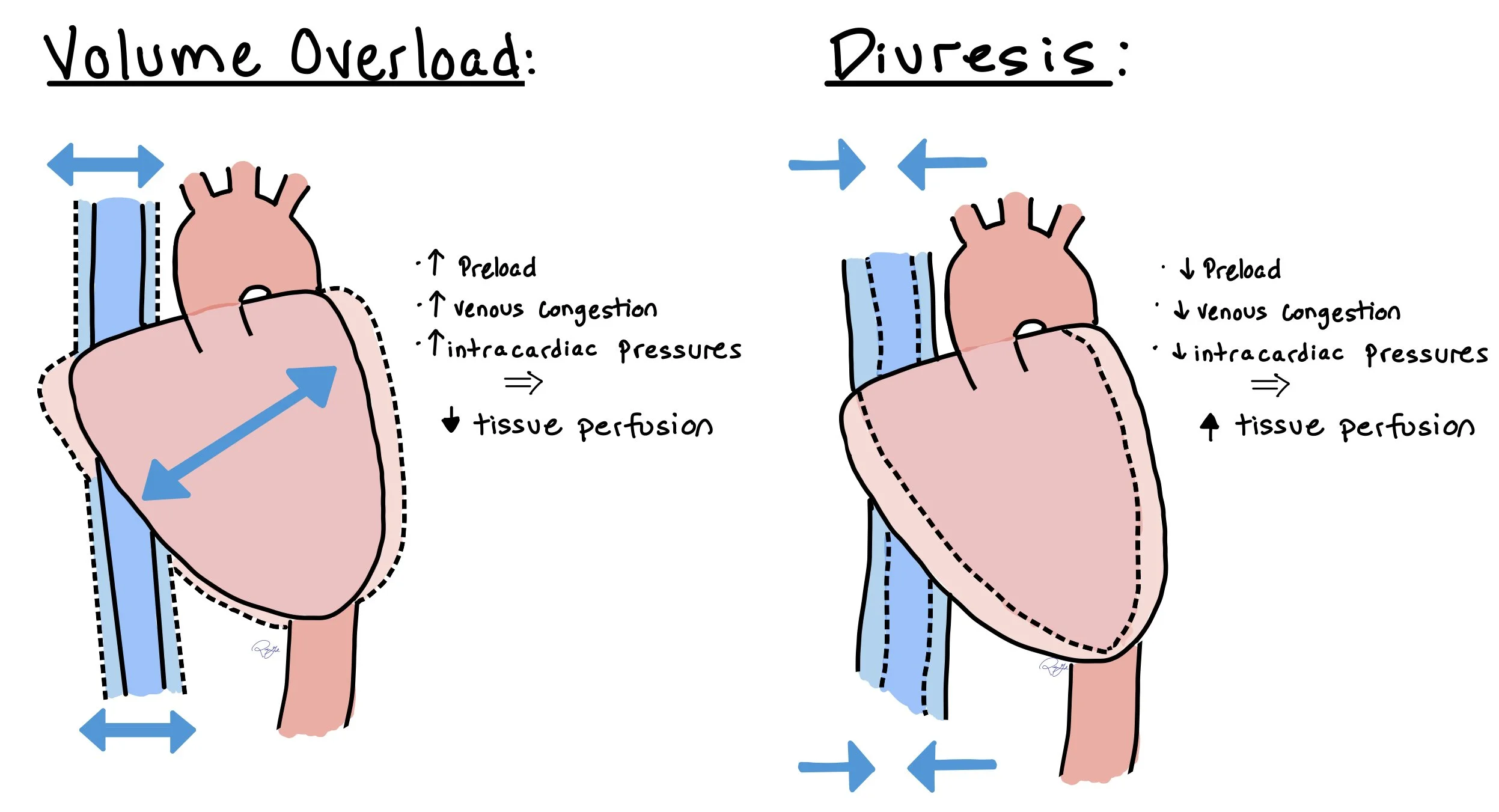
This picture depicts how patients with volume overload benefit from increased diuresis. When overloaded, this increases the preload, venous congestion and intracardiac pressures, causing decreased tissue perfusion. Once diuressed, this allows to help offload the heart by decreasing preload, venous congestion, and intracardiac pressures, which ultimately helps to increase tissue perfusion.
Vasopressors
Vasopressors that should be considered include norepinephrine, epinephrine, and dobutamine. All three provide inotropic support and improvement in MAPs and should be started in patients on an inotrope with refractory cases or anticipated hypotension due to inotropic agent chosen initially. Norepinephrine is considered the vasopressor of choice in patients with cardiogenic shock due to data available showcasing a better safety profile and potentially better outcomes. The mechanism behind norepinephrine’s favorable safety profile and better outcome includes the extent at which it agonizes the alpha-1 and beta receptors compared to other vasopressors (found in chart below). Due to norepinephrine having a high affinity for the alpha-1 receptor it increases MAP and a lower affinity for the beta receptors correlating to a decreased risk for arrhythmias compared to other vasopressors. Vasopressors like phenylephrine and vasopressin should generally be avoided as the first line agent, especially in the absence of concomitant inotropes, since these drugs are pure vasoconstrictors. This produces increases in SVR, which could drop CO further.
Inotropic Agents
The two main inotropes are dobutamine and milrinone. Understanding the key differences between them is crucial for knowing which medication to pick for each patient.
This chart helps to differentiate key differences between Dobutamine and Milrinone, which can help us determine which medication to pick for each individaul patient case.
Considerations for palliative inotropic continuous infusions:
For patients that are eligible for mechanical circulatory support (MCS) or cardiac transplant in stage D HFrEF, inotropes can be continued as bridge therapy. If a Stage D HFrEF patient is not eligible for MCS or transplant, inotropic support as palliative therapy can still be utilized for maintenance of symptom control. If patients do not meet either of these criteria, it is not recommended to continue inotropic support as the risks of adverse events including catheter related infections and arrhythmias which an ICD can help mitigate outweighs the potential benefit of hemodynamic stability.
Inotrope of choice for palliative continuous infusion is milrinone over dobutamine due to data showing a one-year mortality benefit well as ease of initiating/continuing GDMT upon discharge specifically with BB and MRAs. Milrinone also does not have a risk of tachyphylaxis like dobutamine does with prolonged infusions, which is an important consideration when choosing an agent for palliative treatment.
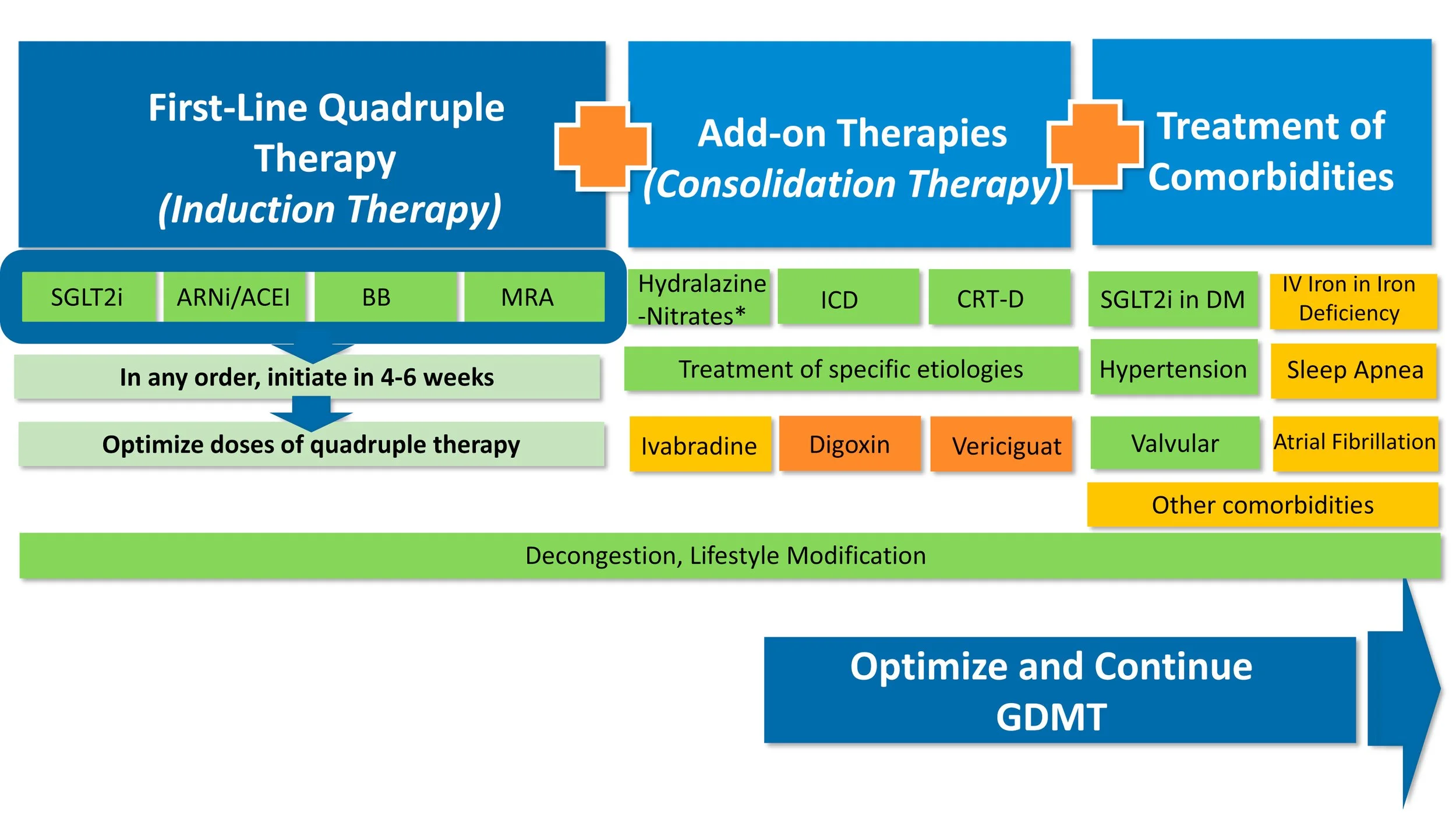
Initiating GDMT
Initiating GDMT in patients admitted for cardiogenic shock (CS):
Patients being admitted for cardiogenic shock with heart failure (HF), should try to be started on GDMT prior to being discharged, although the sequence of initiation should be taken into account considering patients are needing inotropic support. GDMT consists of BB, RAAS-I (ACE-I/ARB/ARNI), MRAs, and SGLT-2 Inhibitors. MRAs and SGLT-2 Inhibitors can be started early on due to their diuresis effect and combating fluid overload in patients presenting with CS as they are likely on diuretics. Caution with initiating BB should only be considered when patients are off inotropic support and are stable off support prior to discharge. ARNIs can sometimes be used to help wean off inotropes. When therapies are initiated, they should be started at low doses to ensure these medications get followed as outpatient as there is data available with initiating BB, RAAS-I, and MRAs prior to discharge decreases one year mortality post-cardiogenic shock (description of study provide in image below).
Typically, we start thinking about heart transplantation or LVAD placement during the index cardiogenic shock admission since we know that mortality long-term after cardiogenic shock is poor. In rare occasions when the patient is too sick to recover, they will get transplanted or LVADs during their index admission. Most of the time though, the patients recover and are closely followed in heart failure clinic. Advanced therapies will then be initiated based on the clinical course.
Mechanical Support
When all else fails, we can use Mechanical Cardiac Support (MCS), which includes the Impella, Balloon Pump, ECMO, Tandem Heart (not pictured), and CentriMag (not pictured). The device we use will depend on each patient’s specific pathology, how much MCS the patient requires, and the device available at each center.
Impella
The Impella uses a motor to help pull blood out from the left ventricle into the aorta. This helps augment cardiac output and decrease cardiac work.
Balloon Pump
The balloon pump uses the inflation and deflation of a balloon to help increase coronary perfusion, decrease cardiac work, and increase cardiac output.
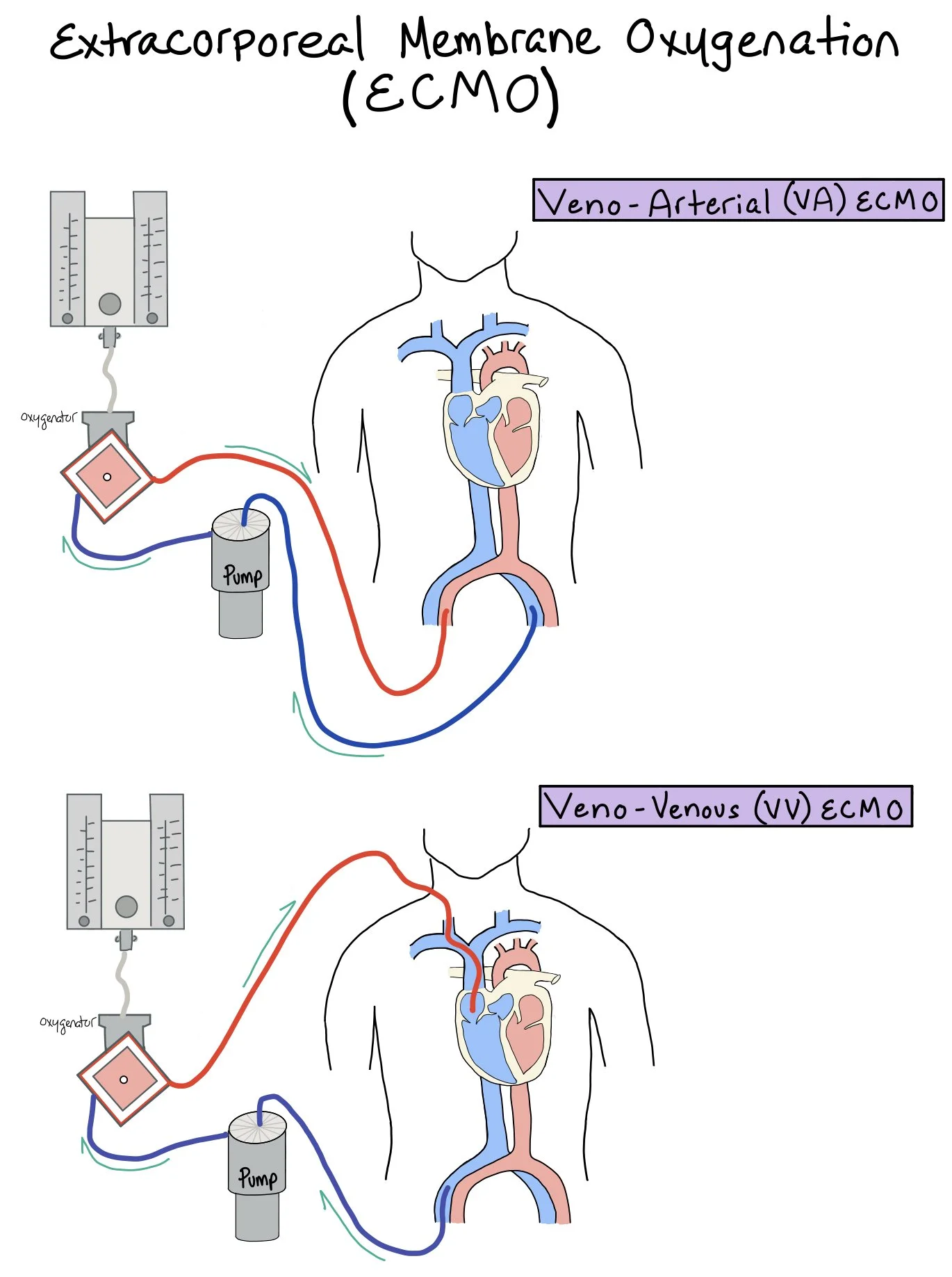
Extracorporeal Membrane Oxygenation (ECMO)
An external machine that helps remove carbon dioxide and pump oxygenated blood to the body. This picture shows a patient on Extracorporeal Membrane Oxygenation (ECMO). Patients will either get veno-arterial (VA) ECMOor veno-venous (VV) ECMO.
** In patients who have mixed cardiogenic shock, there are no randomized studies evaluating MCS options for this shock phenotype. All percutaneous MCS devices can exacerbate inflammation (and thus vasodilation), worsen bleeding risk, and increase risk for infection.
The Pulmonary Artery Catheter aka The Swan:
A PA catheter (AKA Swan-Ganz) can be used to directly measure pressures inside the heart. This catheter is a long, balloon-tipped catheter that gets inserted usually in the internal jugular vein and threads down the SVC into the RA, through the RV, and into the pulmonary artery, where the balloon can be inflated and measure pulmonary capillary wedge pressure, a conduit for left atrial pressure (Figure 5). We expect a different pressure tracing depending on location of the catheter
Thermistor: Intended for thermodilution measurements (CO and CI) as it has a temperature sensitive wire that can help measure temperature change.
Proximal Injection Port: The most proximal port that can be used for infusing medications. Also intended for the cold thermodilution fluid and for measuring central venous pressure (CVP)
Right Atrium (RA) Port: Power plug for the heating filament and used for infusion.
Pulmonary Artery (PA) / Distal Port: Meant for measuring PA pressure and mixed O2 saturation sampling (via VBG)
Balloon Port: Has the balloon attached to the end. This balloon can be inflated up to 1.5mL
This picture depicts waveforms seen as the PA catheter is advanced through the heart. These waveforms are taken from the right atrium (RA) at point A to measure CVP, right ventricle (RV) at point B, pulmonary artery (PA) at point C, and then right after the balloon is inflated (AKA wedged) to correlate to the LA pressures at point D.
Positioning of the Catheter–Should be assessed with CXR daily:
Putting it all together–
Courtesy of Cardio Nerds
Swan Sheet— To be filled out EVERY time a patient has a Swan
Back To The Case:
1. How do you determine what type of shock this patient has? What are you specifically looking for on your labs, imaging, and physical exam to support your assessment?
In many cases, determining the type of shock depends on the clinical presentation, lab values, and imaging. Any time a patient has hypotension that causes poor perfusion and tissue damage, they are in shock. We need to differentiate between cardiogenic, septic, hypovolemic, and obstructive shock. In this case, the patient did not have an overt infection, no signs of acute hemorrhage, or obstruction (D-dimer negative with no signs of heart strain on TTE), ruling out septic, hypovolemic, and obstructive. The patient’s initial symptoms, labs, and imaging could be attributed to acute decompensated heart failure. When the patient was put on a diltiazem drip, he went into cardiogenic shock as it causes decreased chronotropy and HR, thus taking away all cardiac reserve.
We can classify this patient as a Class C Cardiogenic Shock patient presenting with a “cold and wet” presentation secondary to LV dysfunction due to patients past medical history of HF. The RHC numbers also are consistent with cardiogenic shock.
2. What is your initial approach to treating this patient? How do you decide which inotropic agent to treat this patient with?
It should be identified that the cause of this patient’s cardiogenic shock due to the diltiazem being started for afib with RVR in a patient with severe HF, EF: 45% and worsened with the bedside TTE. Diltiazem can induce cardiogenic shock due to its negative inotropic effect with effects seen in more severe heart failure patients due to the contractility of the heart already being impaired in these patients. If this occurs, diltiazem should be discontinued before inotropic agents are started as that is a reversible cause of cardiogenic shock.
The 2 options for inotropic agents for cardiogenic shock include dobutamine or milrinone. This patient should be started on dobutamine over milrinone as patient currently has an AKI and milrinone is primarily renally cleared which could potentiate supratherapeutic levels of milrinone increasing the patients risk of hypotension and arrhythmias. The patient is hypotensive and milrinone has an increased risk of hypotension, so that is additional justification for starting dobutamine. Since this patient is a Class C Cardiogenic Shock utilizing dobutamine is beneficial due to the shorter onset of 1 to 10 minutes compared to 15 minutes with milrinone. The half-life of dobutamine is also significantly shorter than milrinone 2 minutes compared to 2.5 hours indicating dobutamine has a faster time to steady state concentration. Important to note that milrinone could still be utilized in this patient, however, should be started at a lower dose and titrated up as patients renal function improves indicative of an increased CO and organ perfusion. Regardless of the inotrope selected, it is recommended that a pulmonary artery catheter be in place to allow for continuous invasive hemodynamic assessment, which will allow for appropriate titration of the inotrope.
3. What additional therapies should you consider once the patient is stable on their inotropic and vasopressor agents?
While the patient is still requiring inotropes and vasopressors caution on initiating all pillars of GDMT as we do not want to cause hypotension in patients needing support to maintain their blood pressure. Consider initiating SGLT-2 Inhibitors and MRAs as this will help with fluid overload due to their diuretic effect with minimal effect on blood pressure, although renal function should be monitored prior as these medications are renally excreted and due to the lack of perfusion patients may still be in an acute kidney injury and can accumulate drugs. All medications should be initiated on low doses and titrated up. Once the patient no longer needs inotropes or vasopressors and has stable blood pressure other medications including RAAS-I, hydralazine and isosorbide in African American patients, and a selective BB can be considered.
Further Learning:
Resident Responsibilities
Recognizing cardiogenic shock in a timely manner, keep on your differential when a patient is presenting with hypotension and tachycardia
Patients should get IV access and get an EKG to assess for arrhythmias.
Get point of care testing for lactate and BNP to get faster lab values then a blood draw. Do not need to wait for labs to result before treating these will just help you differentiate the extent of the decreased CO. Elevated lactate is indicative of tissue malperfusion (ask patients about UOP, AMS, feel for cold extremities- these are easy to assess organ function without labs). Elevated BNP is indicative of fluid overload look for JVD, pitting edema, and crackles in the lung.
Think about what inotropic agent to choose based on patient specific factors to guide decision making
In addition to inotropes, vasopressors should be added on to get to MAP goals (>65) as inotropes can further decrease BP. Norepinephrine is recommended as the first line vasopressor for most patients with left ventricular cardiogenic shock.
Don’t forget to treat the volume overload with IV loop diuretic therapy! And if the patient is not responding to initial doses, do not hesitate to increase the dose, consider loop diuretic infusions, or add additional agents for sequential nephron blockade.
Make sure prior to discharge to initiate GDMT once patient is stable to help prevent readmissions
Further Reading
“Hemodynamics for the Heart Failure Clinician”: Excellent overview of interpretation of PA catheter tracings
CardioNerds: Right Heart Catheterization
DanGer Shock: Trial evaluating patients with STEMI and cardiogenic shock with patients who received an Impella. These patients had reduction in 180-day all cause mortality when compared to standard care alone (although it was also associated with greater risk of some adverse events).
Palliative Inotrope Review: Comparing outcomes between Milrinone and Dubutamine by Sami et al
How’d we do?
The following individuals contributed to this topic: Laila Hammad, PharmD; Chelsea Tweneboah, MD; Stephanie Dwyer, PharmD; Orly Levia, MD
Chapter Resources
Ranka S, Mastoris I, Kapur NK, et al. Right Heart Catheterization in Cardiogenic Shock Is Associated With Improved Outcomes: Insights From the Nationwide Readmissions Database. J Am Heart Assoc. 2021;10(17):e019843. doi:10.1161/JAHA.120.019843
Eric L. Bonno, Michael C. Viray, Gregory R. Jackson, Brian A. Houston, Ryan J. Tedford; Modern Right Heart Catheterization: Beyond Simple Hemodynamics. Advances in Pulmonary Hypertension 1 January 2020; 19 (1): 6–15. doi: https://doi.org/10.21693/1933-088X-19.1.6
Vahdatpour C, Collins D, Goldberg S. Cardiogenic Shock. J Am Heart Assoc. 2019;8(8):e011991. doi:10.1161/JAHA.119.011991
Naidu SS, Baran DA, Jentzer JC, et al. SCAI SHOCK Stage Classification Expert Consensus Update: A Review and Incorporation of Validation Studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J Am Coll Cardiol. 2022;79(9):933-946. doi:10.1016/j.jacc.2022.01.018
Sarma D, Jentzer JC. Cardiogenic Shock: Pathogenesis, Classification, and Management. Crit Care Clin. 2024;40(1):37-56. doi:10.1016/j.ccc.2023.05.001
Alkhunaizi FA, Smith N, Brusca SB, Furfaro D. The Management of Cardiogenic Shock From Diagnosis to Devices: A Narrative Review. CHEST Crit Care. 2024;2(2):100071. doi:10.1016/j.chstcc.2024.100071
Leier CV, Webel J, Bush CA. The cardiovascular effects of the continuous infusion of dobutamine in patients with severe cardiac failure. Circulation. 1977;56(3):468-472. doi:10.1161/01.cir.56.3.468
Milrinone. In: Lexi-Drugs. Lexidrug, Inc. Updated February 8, 2025. Accessed February 27, 2025.
Dobutamine. In: Lexi-Drugs. Lexidrug, Inc. Updated February 25, 2025. Accessed February 27, 2025.
Meekers E, Dauw J, Ter Maaten JM, et al. Urinary sodium analysis: The key to effective diuretic titration? European Journal of Heart Failure expert consensus document. Eur J Heart Fail. Published online February 27, 2025. doi:10.1002/ejhf.3632
Overgaard CB, Dzavík V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. Circulation. 2008;118(10):1047-1056. doi:10.1161/CIRCULATIONAHA.107.728840
Narang N, Blumer V, Jumean MF, et al. Management of Heart Failure-Related Cardiogenic Shock: Practical Guidance for Clinicians. JACC Heart Fail. 2023;11(7):845-851. doi:10.1016/j.jchf.2023.04.010
Bloom JE, Chan W, Kaye DM, Stub D. State of Shock: Contemporary Vasopressor and Inotrope Use in Cardiogenic Shock. J Am Heart Assoc. 2023;12(15):e029787. doi:10.1161/JAHA.123.029787
Bozkurt B. How to Initiate and Uptitrate GDMT in Heart Failure: Practical Stepwise Approach to Optimization of GDMT. JACC Heart Fail. 2022;10(12):992-995. doi:10.1016/j.jchf.2022.10.005
Matsushita K, Delmas C, Marchandot B, et al. Optimal Heart Failure Medical Therapy and Mortality in Survivors of Cardiogenic Shock: Insights From the FRENSHOCK Registry. J Am Heart Assoc. 2024;13(5):e030975. doi:10.1161/JAHA.123.030975
Sami F, Acharya P, Noonan G, et al. Palliative Inotropes in Advanced Heart Failure: Comparing Outcomes Between Milrinone and Dobutamine. J Card Fail. 2022;28(12):1683-1691. doi:10.1016/j.cardfail.2022.08.007
Packer M. Calcium channel blockers in chronic heart failure. The risks of "physiologically rational" therapy. Circulation. 1990;82(6):2254-2257. doi:10.1161/01.cir.82.6.2254
Mathew R, Di Santo P, Jung RG, et al. Milrinone as Compared with Dobutamine in the Treatment of Cardiogenic Shock. N Engl J Med. 2021;385(6):516-525. doi:10.1056/NEJMoa2026845
Biswas S, Malik AH, Bandyopadhyay D, et al. Meta-analysis Comparing the Efficacy of Dobutamine Versus Milrinone in Acute Decompensated Heart Failure and Cardiogenic Shock. Curr Probl Cardiol. 2023;48(8):101245. doi:10.1016/j.cpcardiol.2022.101245