Case Presentation:
A 58-year-old man with a past medical history of HFrEF (EF ~ 25%), type 2 diabetes mellitus (A1c 10.8%), and CKD stage 3 presents to the ED with tachypnea, nausea, vomiting, and abdominal pain. He reports decreased oral intake over the past few days due to an intercurrent viral illness. His medications include metformin, empagliflozin, carvedilol, sacubitril-valsartan, and spironolactone.
Vitals: Febrile to 101 F, BP 110/70s, HR 120s, RR 20s, saturating 92% on room air
Initial labs include a basic metabolic panel, which reveals an elevated serum creatinine of 2.6 mg/dL (baseline ~ 1.6), serum glucose of 150 mg/dL, a low serum bicarbonate of 15 mEq/L, and an anion gap of 20. A subsequent ABG shows a pH of 7.27, with pCO2 of 30 mmHg. Urinalysis is positive for ketones and glucose.
Ask Yourself:
What is the clinical approach to treating this patient?
What are the clinical indications and contraindications for SGLT2 inhibitor use?
How can starvation ketosis be differentiated from euglycemic DKA?
After an episode of euglycemic DKA or severe ketosis, when can SGLT2 inhibitors be safely restarted?
What strategies can help reduce the risk of SGLT2i-associated complications like DKA?
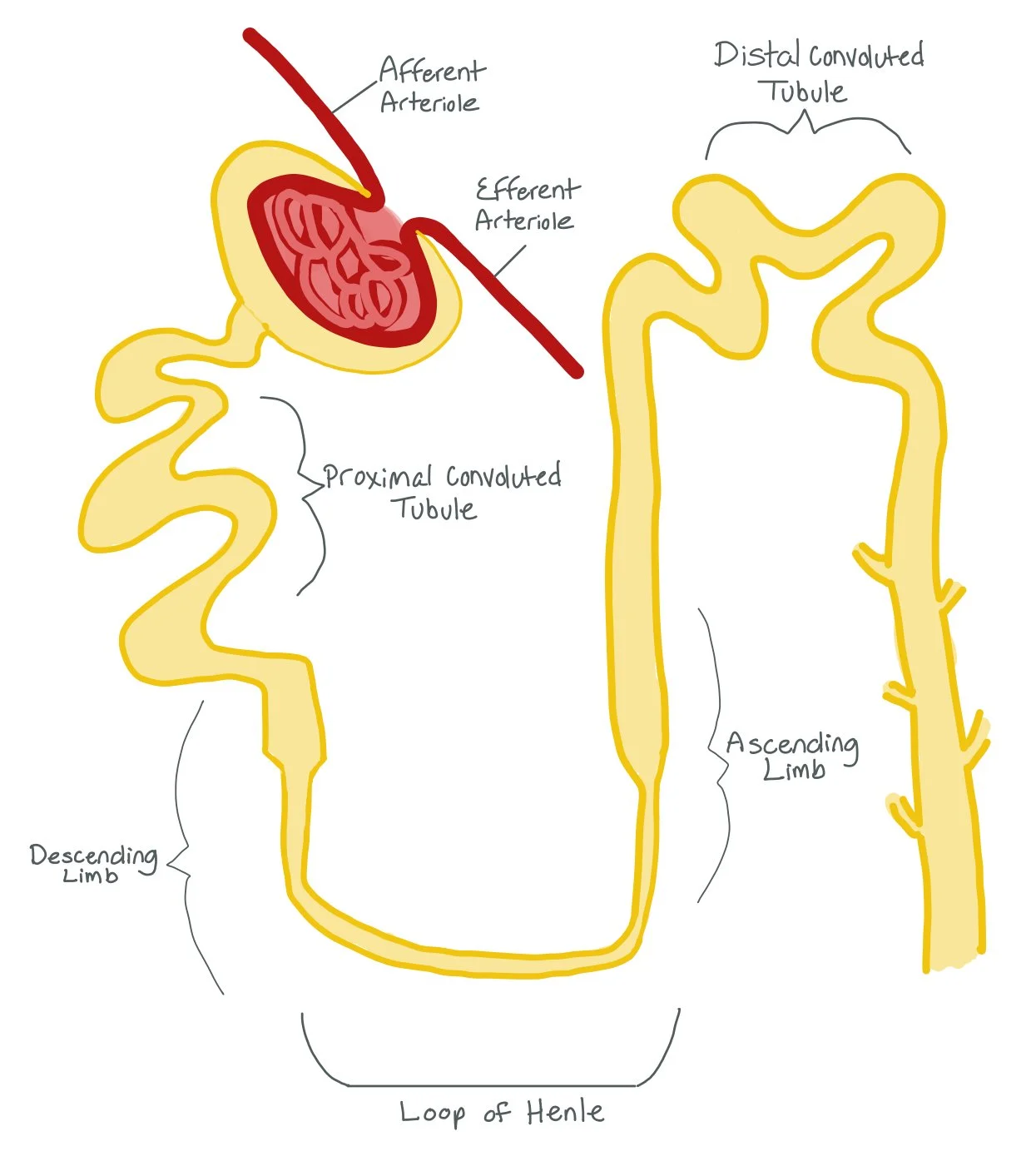
The picture above shows the different parts of the nephron, from the Afferent and Efferent Arterials, the Proximal Convoluted Tubule (PCT), the Descending and Ascending Limbs of the Loop of Henle, and the Distal Convoluted Tubule (DCT).
Background:
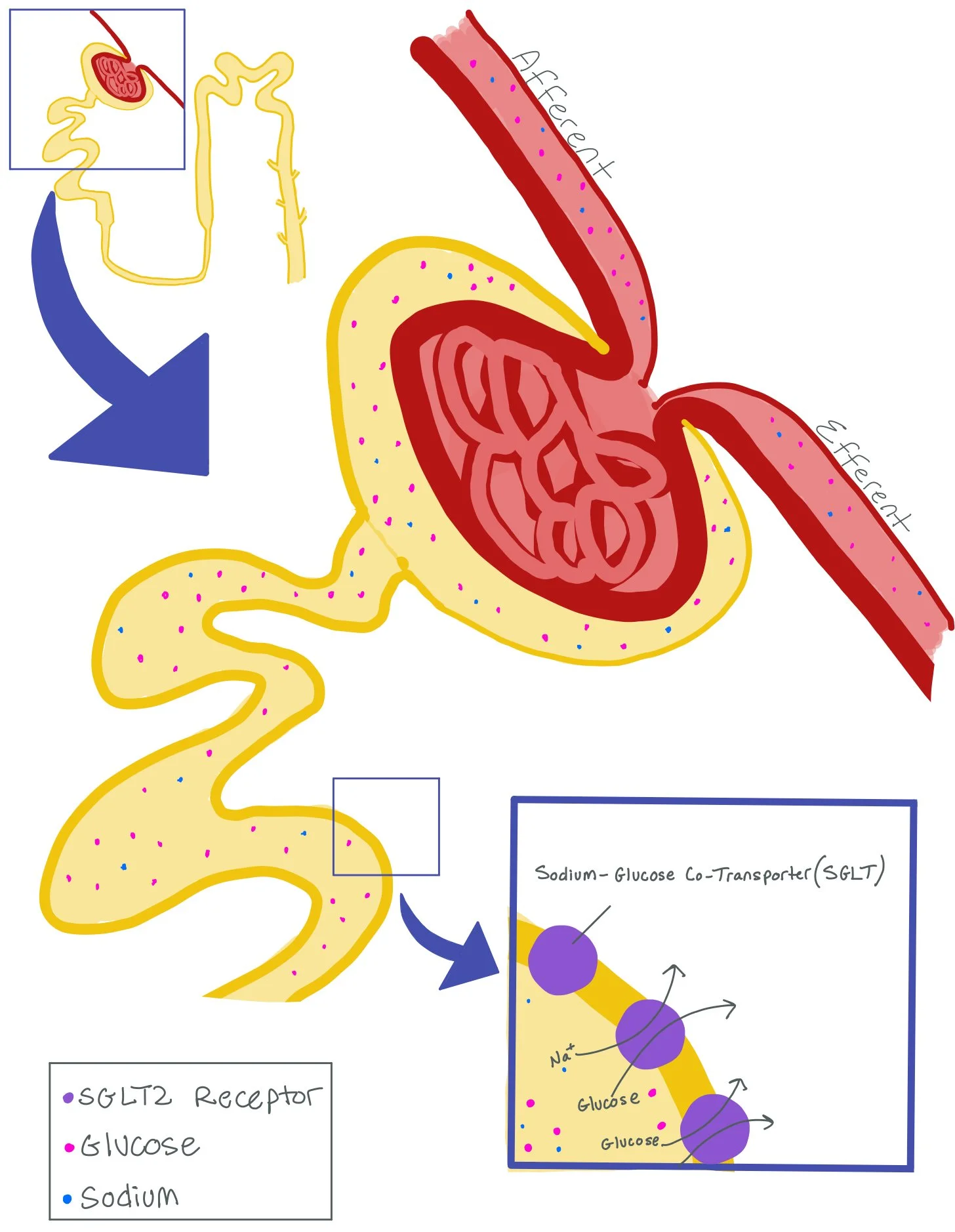
Sodium-Glucose Co-Transporter 2 inhibitors (SGLT2i) are hypoglycemic agents that function as inhibitors of the SGLT2 receptor in the proximal renal tubules. Through the inhibition of the receptor, they reduce the reabsorption of glucose and promote urinary excretion of glucose. SGLT2is include drugs like empagliflozin, dapagliflozin, canagliflozin, and ertugliflozin.
In this picture, we are focusing on the Afferent and Efferent Arteriole, as well as the Proximal Convoluted Tubule (PCT). Note how the PCT has a Sodium-Glucose Co-Transporter (SGLT) Receptor. While there are both SGLT1 and SGLT2 receptors, the majority of receptors are SGLT2 receptors. These receptors put both sodium and glucose back into the bloodstream after it is brought into the nephron.
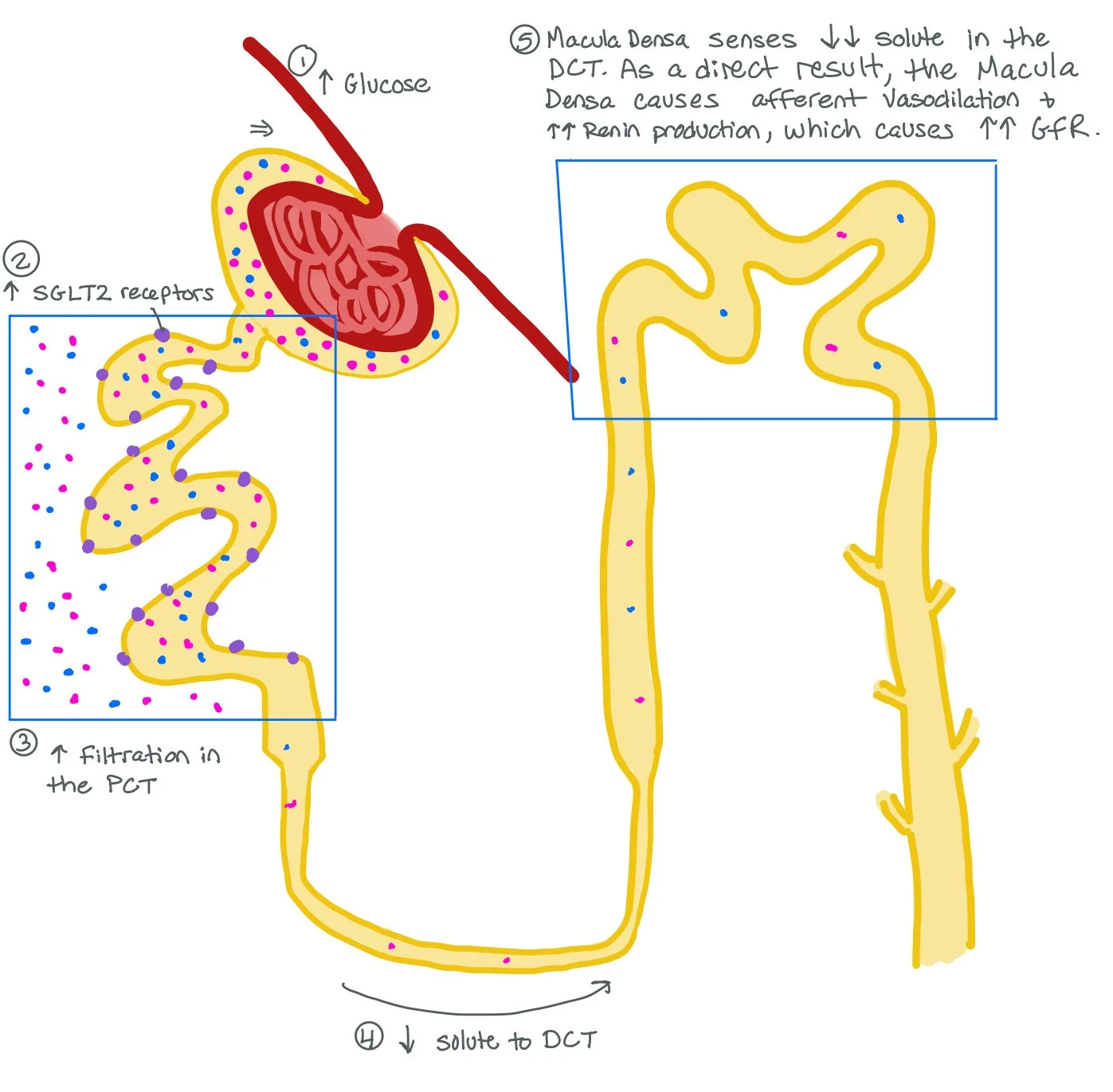
This picture shows how more sugar in the bloodstream increases the amount of SGLT2 receptors in the PCT. As a direct result, most of the solute gets put back into the bloodstream in the PCT and LESS solute in the Distal Convoluted Tubule (DCT).
The Macula Densa senses the decreased amount of solute in the DCT and will cause vasodilation of the Afferent Arteriole and increased RAAS production, which will cause vasoconstriction of the Efferent Arteriole.
All together, the increased SGLT2 receptors, vasodilation of the Afferent Arteriole, and vasoconstriction of the Efferent Arteriole cause increased filtration through the nephron. This increased filtration rate can cause a hyperfiltration injury to the nephron.
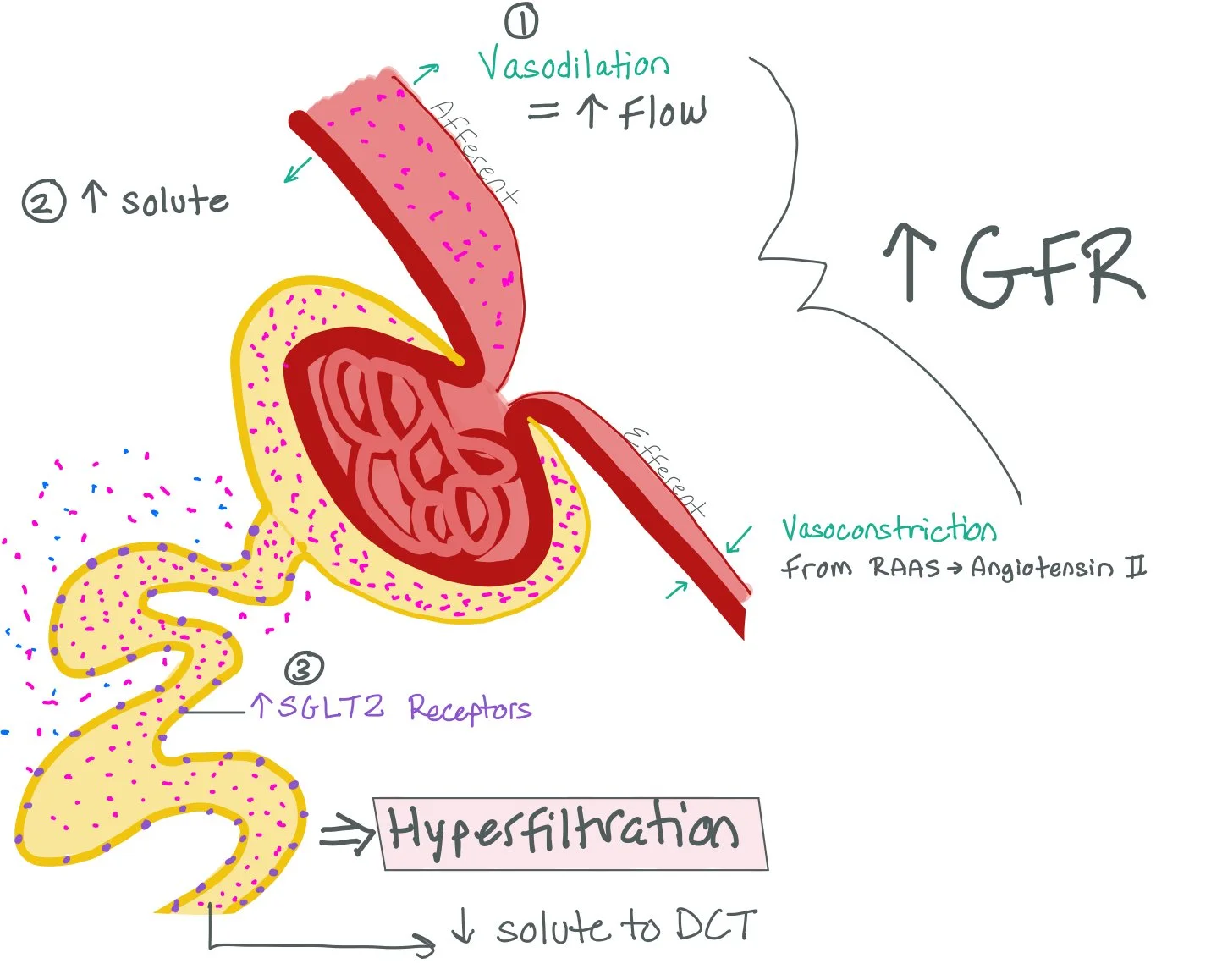
The Macula Densa senses the decreased amount of solute in the DCT and will cause vasodilation of the Afferent Arteriole and increased RAAS production, which will cause vasoconstriction of the Efferent Arteriole.
All together, the increased SGLT2 receptors, vasodilation of the Afferent Arteriole, and vasoconstriction of the Efferent Arteriole cause increased filtration through the nephron. This increased filtration rate can cause a hyperfiltration injury to the nephron.
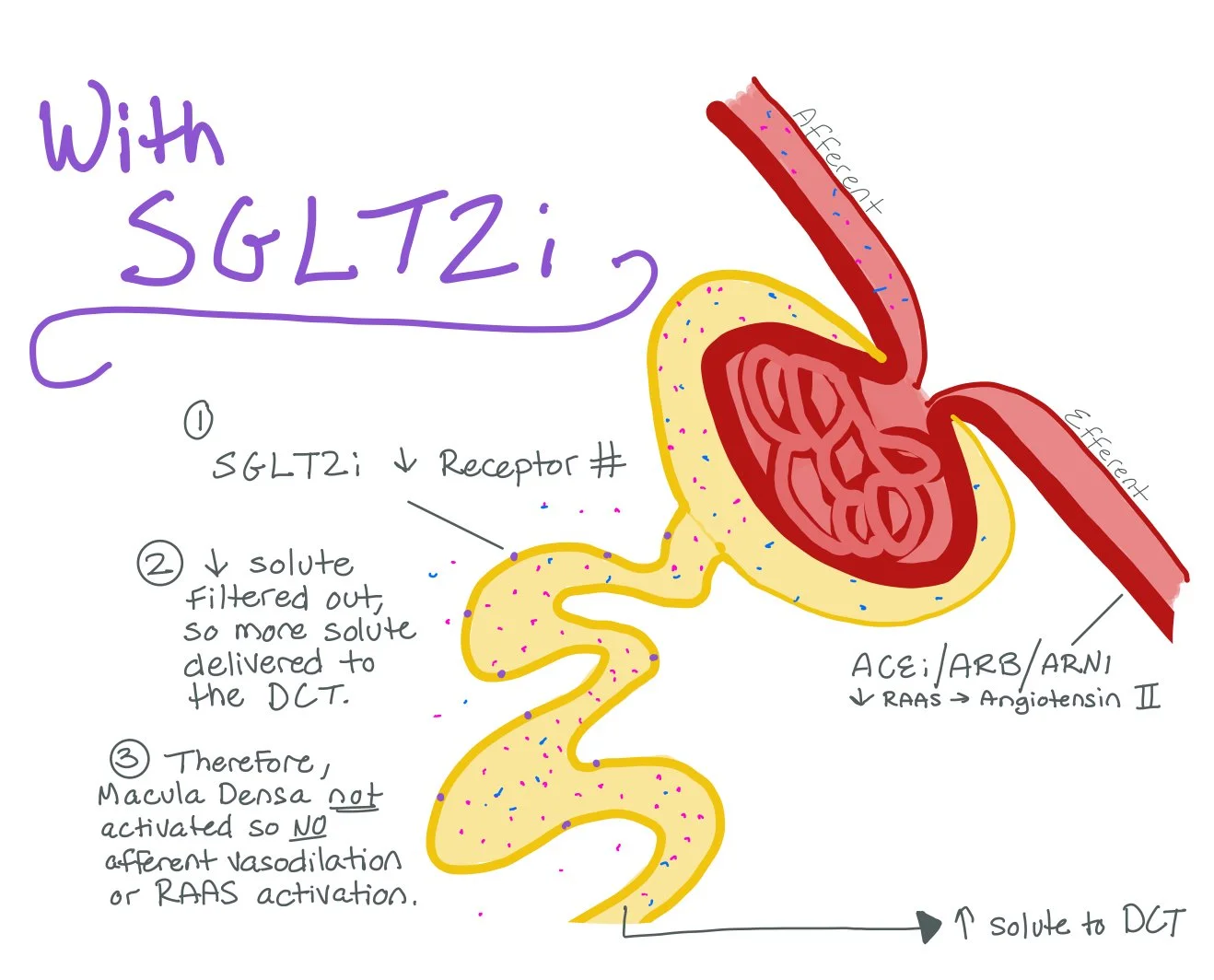
SGLT2 inhibitors will prevent the body from making more SGLT2 receptors. As a result, the PCT will be unable to filter more solute back into the bloodstream. This will allow more solute to make it to the DCT, which will prevent the Macula Densa from vasodialating the Afferent Arteriole and producing increased RAAS for Efferent Arteriole vasoconstriction.
Ultimately, SGLT2i work by preventing hyperfiltration injury.
Major Indications and Contraindications:
Major Indications:
Type 2 Diabetes Mellitus: Improves glycemic control and reduces cardiovascular events (EMPA-REG OUTCOME).
Heart Failure with Reduced Ejection Fraction: Reduces heart failure hospitalizations and cardiovascular death (DAPA-HF, EMPEROR-Reduced).
Heart Failure with Preserved Ejection Fraction: Reduces heart failure hospitalizations and improves outcomes (EMPEROR-Preserved).
Chronic Kidney Disease: Slows progression of kidney disease and reduces cardiovascular events (DAPA-CKD).
Nonalcoholic fatty liver disease (NAFLD): Recommended by the American Association of Clinical Endocrinology (AACE) as adjunctive treatment for NAFLD in patients with T2DM.
The table above summarizes the major clinical trials investigating SGLT2 inhibitors in cardiovascular, heart failure, and kidney disease outcomes. HHF: Hospitalization for Heart Failure; MACE: Major Adverse Cardiac Events.
Major Contraindications:
Absolute Contraindications:
Active diabetic ketoacidosis (DKA)
Severe renal impairment (***eGFR < 20–25 mL/min/1.73m² depending on the agent and indication***)
Active or history of Fournier’s gangrene
Pregnancy
Type 1 Diabetes Mellitus
Relative Contraindications/Cautions:
History of DKA - see the section “When to Restart SGLT2 Inhibitors?” below
Bariatric surgery: increases risk of euglycemic diabetic ketoacidosis (DKA) post-surgery due to decreased caloric intake, decreased intake of carbohydrates, dehydration, and/or decreased insulin dose post-procedure
Malnutrition or low-carb diet
Volume depletion or hypotension at baseline
Severe active infections, especially genitourinary
Hospitalized patients: not routinely recommended for glycemic control (ADA 2023).
Recognizing and Differentiating Euglycemic DKA
Euglycemic DKA (EDKA) is DKA with glucose levels <200 mg/dL, often triggered by SGLT2 inhibitors. Clinical signs are similar to those of DKA, such as nausea, vomiting, abdominal pain, tachypnea, confusion. Key labs include a high anion gap metabolic acidosis and elevated serum and urine ketones. Unlike DKA, patients with EDKA present with normal or mildly elevated glucose.
Starvation ketosis can mimic EDKA but usually presents with:
Mild acidosis.
Normal to low glucose.
Minimal dehydration.
Absence of significant hyperketonemia compared to DKA.
Absence or minimal amounts of glucose in the urine.
Clue: Positive urine glucose supports recent SGLT2i use and possible EDKA over starvation ketosis.
The table above summarizes the difference between starvation ketosis and euglycemic DKA.
*While EDKA often presents with significant acidosis and ketonemia, milder forms can occur — especially early in the disease course or in well-compensated patients.
When to Restart SGLT2 Inhibitors?
Restarting SGLT2i after an episode of DKA (or suspected DKA) is nuanced. When considering re-initiation, ask yourself the following questions:
Was DKA clearly related to transient factors (e.g., acute illness, fasting)? If yes, and these are resolved, SGLT2i may be cautiously restarted.
Was DKA spontaneous and without a clear precipitant? Does the patient repeatedly present with recurrent episodes of DKA despite adequate precautions? If yes, consider permanently discontinuing the SGLT2i.
Regardless of the situation, shared decision-making with the patient is crucial. Weighing the cardiovascular and renal benefits against the risk of recurrence may differ due to patient-specific factors. Of note, some evidence suggests that the absolute benefits of SGLT2i initiation (i.e, cardiovascular outcomes) significantly outweigh serious hazards such as ketoacidosis (Refer to Resources [15] for the full meta-analysis).
Preventing SGLT2i-Associated DKA
Sick Day Rules & Emergency Situations: Hold SGLT2i during periods of illness, surgery, volume depletion, or fasting.
Perioperative Management: Discontinue SGLT2i 3–4 days before elective surgeries or invasive procedure. You may restart the medication after oral intake returns to baseline and risk factors for DKA resolve.
Hydration: Encourage good oral hydration, especially during illness or demanding activities and severe physical exertion.
Patient Education: Educate patients on early signs of DKA (nausea, vomiting, rapid breathing) even with normal glucose. Counsel patients to avoid excessive alcohol intake and low-carb/ketogenic diets.
Close Monitoring: Low threshold to check ketones if patients develop symptoms suggestive of DKA.
Insulin Therapy: Do not stop insulin or lower the dose by more than 20% when initiating an SGLT2i in patients who are insulin-dependent, such as patients with T1DM or pancreatogenic diabetes.
Back to the Case:
1. What is the clinical approach to treating this patient?
Many times, euglygemic DKA can be masked by other symptoms or presumed diagnoses. Given the fact that many of our cardiac patients on are SGLT2is, it’s important for us to have a high index of suspicion for euglycemic DKA. Paying particular attention to the bicarb, anion gap, ABG, and UA can help us with our differential.
2. What are the clinical indications and contraindications for SGLT2 inhibitor use?
Major indications for SGLT2i are HFrEF, HFpEF, diabetes type 2, CKD, and NAFLD. There are both absolute and relative contraindications of use. The absolute contraindications include active diabetic ketoacidosis (DKA), severe renal impairment, active or history of Fournier’s gangrene, pregnancy, and Type 1 Diabetes Mellitus
3. How can starvation ketosis be differentiated from euglycemic DKA?
Starvation ketosis can mimic EDKA but usually presents with mild acidosis, normal to low glucose, minimal dehydration. absence of significant hyperketonemia compared to DKA, and absence or minimal amounts of glucose in the urine.
The way we distinguish the two is by looking at the urine glucose!! Positive urine glucose supports recent SGLT2i use and possible EDKA over starvation ketosis as patients in starvation ketosis do not have enough glucose (hence the starvation).
4. After an episode of euglycemic DKA or severe ketosis, when can SGLT2 inhibitors be safely restarted?
Restarting SGLT2i after an episode of DKA (or suspected DKA) is nuanced. When considering re-initiation, ask yourself the following questions:
Was DKA clearly related to transient factors (e.g., acute illness, fasting)? If yes, and these are resolved, SGLT2i may be cautiously restarted.
Was DKA spontaneous and without a clear precipitant? Does the patient repeatedly present with recurrent episodes of DKA despite adequate precautions? If yes, consider permanently discontinuing the SGLT2i.
Regardless of the situation, shared decision-making with the patient is crucial. Weighing the cardiovascular and renal benefits against the risk of recurrence may differ due to patient-specific factors. Of note, some evidence suggests that the absolute benefits of SGLT2i initiation (i.e, cardiovascular outcomes) significantly outweigh serious hazards such as ketoacidosis (Refer to Resources [15] for the full meta-analysis).
5. What strategies can help reduce the risk of SGLT2i-associated complications like DKA?
Consider holding SGLT2i when patients are sick or may be fasting (think preoperatively). When patients are on the medication, ensure they have good hydration and are educated to look for signs of early DKA (nausea, vomiting, rapid breathing) even with normal glucose. Counsel patients to avoid excessive alcohol intake and low-carb/ketogenic diets.
Further Learning:
Resident Responsibilities:
Always keep euglycemic DKA in the back of your mind for patients who are on SGLT2i! Just because the glucose is not high does not mean the patient is not in DKA!
Make sure to appropriately counsel your patients regarding when they should take or hold their SGLT2is, such as when they are sick, may be fasting, or have poor PO intake. This will help prevent euglycemic DKA.
It is also crucial you tell your patients what to expect in case they have euglycemic DKA, such as nausea, vomiting, tachypnea, and dehydration.
If you think a patient does have euglycemic DKA, make sure to rule out starvation ketosis by getting a UA to see if there is glucose in the urine!
Further Reading:
EMPA-REG OUTCOME Trial: Demonstrated cardiovascular benefit of empagliflozin in patients with T2DM and established cardiovascular disease.
EMPA-KIDNEY Trial: Showed that empagliflozin reduces the risk of kidney disease progression or death from cardiovascular causes in patients with CKD even without diabetes.
This is a great article reviewing the different major trials that studied SGLT2i.
How’d we do?
The following individuals contributed to this topic: Nadia Sweis, MD, Julienne Sanchez Perez, MD; Stephanie Dwyer Kaluza, PharmD, BCCP; Yuval Eisenberg, MD
Chapter Resources
Padda IS, Mahtani AU, Parmar M. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors. [Updated 2023 Jun 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK576405/
EMPA-REG OUTCOME (2015)
Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/NEJMoa1504720
CANVAS Program (2017)
Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657. doi:10.1056/NEJMoa1611925
DECLARE–TIMI 58 (2019)
Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357. doi:10.1056/NEJMoa1812389
VERTIS-CV (2020)
Cannon CP, Pratley R, Dagogo-Jack S, et al; VERTIS-CV Investigators. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383(15):1425-1435. doi:10.1056/NEJMoa2004967
DAPA-HF (2019)
McMurray JJV, Solomon SD, Inzucchi SE, et al; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008. doi:10.1056/NEJMoa1911303
EMPEROR-Reduced (2020)
Packer M, Anker SD, Butler J, et al; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. doi:10.1056/NEJMoa2022190
EMPEROR-Preserved (2021)
Anker SD, Butler J, Filippatos G, et al; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. doi:10.1056/NEJMoa2107038
DELIVER (2022)
Solomon SD, McMurray JJV, Claggett B, et al; DELIVER Trial Committees and Investigators. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089-1098. doi:10.1056/NEJMoa2206286
CREDENCE (2019)
Perkovic V, Jardine MJ, Neal B, et al; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306. doi:10.1056/NEJMoa1811744
DAPA-CKD (2020)
Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436-1446. doi:10.1056/NEJMoa2024816
EMPA-KIDNEY (2022)
The EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127. doi:10.1056/NEJMoa2204233
Cusi K, Isaacs S, Barb D, et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr Pract. 2022;28(5):528-562. doi:10.1016/j.eprac.2022.03.010
MacIsaac RJ, Jerums G, Ekinci EI. Harms and benefits of sodium-glucose co-transporter 2 inhibitors. Aust Prescr.2020;43(5):151-154. doi:10.18773/austprescr.2020.049
Devineni D, Morrow L. Empagliflozin: Drug information. UpToDate. Waltham, MA: UpToDate Inc. [Accessed April 29, 2025]. Available from: https://www.uptodate.com
Barski L, Nevzorov R, Jotkowitz A, Rabaev E. Diabetic ketoacidosis in adults: Clinical features, evaluation, and diagnosis. UpToDate. Waltham, MA: UpToDate Inc. [Accessed April 29, 2025]. Available from: https://www.uptodate.com
Umpierrez GE, Davis GM, ElSayed NA, et al. Hyperglycemic Crises in Adults With Diabetes: A Consensus Report. Diabetes Care. 2024;47(8):1257-1275. doi:10.2337/dci24-0032
American Diabetes Association. 16. Diabetes care in the hospital: Standards of Care in Diabetes—2023. Diabetes Care. 2023;46(Suppl 1):S254-S263. doi:10.2337/dc23-S016
Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists' Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788-1801. doi:10.1016/S0140-6736(22)02074-8
Liew A, Lydia A, Matawaran BJ, Susantitaphong P, Tran HTB, Lim LL. Practical considerations for the use of SGLT-2 inhibitors in the Asia-Pacific countries-An expert consensus statement. Nephrology (Carlton). 2023;28(8):415-424. doi:10.1111/nep.14167